Core promoter activity contributes to chromatin-based regulation of internal cryptic promoters
- PMID: 34320189
- PMCID: PMC8373055
- DOI: 10.1093/nar/gkab639
Core promoter activity contributes to chromatin-based regulation of internal cryptic promoters
Abstract
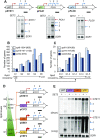
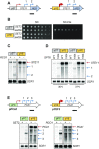
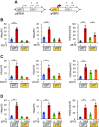
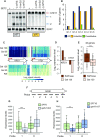
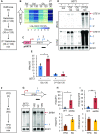
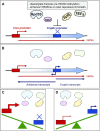
During RNA polymerase II (RNA Pol II) transcription, the chromatin structure undergoes dynamic changes, including opening and closing of the nucleosome to enhance transcription elongation and fidelity. These changes are mediated by transcription elongation factors, including Spt6, the FACT complex, and the Set2-Rpd3S HDAC pathway. These factors not only contribute to RNA Pol II elongation, reset the repressive chromatin structures after RNA Pol II has passed, thereby inhibiting aberrant transcription initiation from the internal cryptic promoters within gene bodies. Notably, the internal cryptic promoters of infrequently transcribed genes are sensitive to such chromatin-based regulation but those of hyperactive genes are not. To determine why, the weak core promoters of genes that generate cryptic transcripts in cells lacking transcription elongation factors (e.g. STE11) were replaced with those from more active genes. Interestingly, as core promoter activity increased, activation of internal cryptic promoter dropped. This associated with loss of active histone modifications at the internal cryptic promoter. Moreover, environmental changes and transcription elongation factor mutations that downregulated the core promoters of highly active genes concomitantly increased their cryptic transcription. We therefore propose that the chromatin-based regulation of internal cryptic promoters is mediated by core promoter strength as well as transcription elongation factors.
© The Author(s) 2021. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

