Open-label follow-on study evaluating the efficacy, safety, and quality of life with extended daily oral immunotherapy in children with peanut allergy
- PMID: 34320250
- PMCID: PMC9293305
- DOI: 10.1111/all.15027
Open-label follow-on study evaluating the efficacy, safety, and quality of life with extended daily oral immunotherapy in children with peanut allergy
Abstract
Background: The benefit of daily administration of Peanut (Arachis hypogaea) Allergen Powder-dnfp (PTAH)-formerly AR101-has been established in clinical trials, but limited data past the first year of treatment are available. This longitudinal analysis aimed to explore the impact of continued PTAH therapeutic maintenance dosing (300 mg/day) on efficacy, safety/tolerability, and food allergy-related quality of life.
Methods: We present a subset analysis of PALISADE-ARC004 participants (aged 4-17 years) who received 300 mg PTAH daily for a total of ~1.5 (Group A, n = 110) or ~2 years (Group B, n = 32). Safety assessments included monitoring the incidence of adverse events (AEs), accidental exposures to food allergens, and adrenaline use. Efficacy was assessed by double-blind, placebo-controlled food challenge (DBPCFC); skin prick testing; peanut-specific antibody assays; and Food Allergy Quality of Life Questionnaire (FAQLQ) and Food Allergy Independent Measure (FAIM) scores.
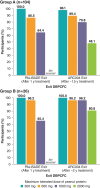
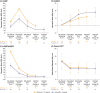
Results: Continued maintenance with PTAH increased participants' ability to tolerate peanut protein: 48.1% of completers in Group A (n = 50/104) and 80.8% in Group B (n = 21/26) tolerated 2000 mg peanut protein at exit DBPCFC without dose-limiting symptoms. Immune biomarkers showed a pattern consistent with treatment-induced desensitization. Among PTAH-continuing participants, the overall and treatment-related exposure-adjusted AE rate decreased throughout the intervention period in both groups. Clinically meaningful improvements in FAQLQ and FAIM scores over time suggest a potential link between increased desensitization as determined by the DBPCFC and improved quality of life.
Conclusions: These results demonstrate that daily PTAH treatment for peanut allergy beyond 1 year leads to an improved safety/tolerability profile and continued clinical and immunological response.
Trial registration: ClinicalTrials.gov NCT03201003 NCT02635776 NCT03292484.
Keywords: desensitization; food allergy; oral immunotherapy; peanut allergy; quality of life.
© 2021 Aimmune Therapeutics. Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
Figures



References
-
- Nwaru BI, Hickstein L, Panesar SS, et al. Prevalence of common food allergies in Europe: a systematic review and meta‐analysis. Allergy 2014;69(8):992‐1007. - PubMed
-
- Vereda A, van Hage M, Ahlstedt S, et al. Peanut allergy: Clinical and immunologic differences among patients from 3 different geographic regions. J Allergy Clin Immunol 2011;127(3):603‐607. - PubMed

