Maresin 1 activates LGR6 signaling to inhibit smooth muscle cell activation and attenuate murine abdominal aortic aneurysm formation
- PMID: 34320253
- PMCID: PMC9170188
- DOI: 10.1096/fj.202100484R
Maresin 1 activates LGR6 signaling to inhibit smooth muscle cell activation and attenuate murine abdominal aortic aneurysm formation
Abstract
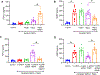
The specialized pro-resolving lipid mediator maresin 1 (MaR1) is involved in the resolution phase of tissue inflammation. It was hypothesized that exogenous administration of MaR1 would attenuate abdominal aortic aneurysm (AAA) growth in a cytokine-dependent manner via LGR6 receptor signaling and macrophage-dependent efferocytosis of smooth muscle cells (SMCs). AAAs were induced in C57BL/6 wild-type (WT) mice and smooth muscle cell specific TGF-β2 receptor knockout (SMC-TGFβr2-/- ) mice using a topical elastase AAA model. MaR1 treatment significantly attenuated AAA growth as well as increased aortic SMC α-actin and TGF-β2 expressions in WT mice, but not SMC-TGFβr2-/- mice, compared to vehicle-treated mice. In vivo inhibition of LGR6 receptors obliterated MaR1-dependent protection in AAA formation and SMC α-actin expression. Furthermore, MaR1 upregulated macrophage-dependent efferocytosis of apoptotic SMCs in murine aortic tissue during AAA formation. In vitro studies demonstrate that MaR1-LGR6 interaction upregulates TGF-β2 expression and decreases MMP2 activity during crosstalk of macrophage-apoptotic SMCs. In summary, these results demonstrate that MaR1 activates LGR6 receptors to upregulate macrophage-dependent efferocytosis, increases TGF-β expression, preserves aortic wall remodeling and attenuate AAA formation. Therefore, this study demonstrates the potential of MaR1-LGR6-mediated mitigation of vascular remodeling through increased efferocytosis of apoptotic SMCs via TGF-β2 to attenuate AAA formation.
Keywords: aneurysm; aorta; efferocytosis; macrophage; maresin; smooth muscle cells; transforming growth factor beta 2.
© 2021 Federation of American Societies for Experimental Biology.
Figures





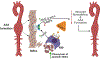
References
-
- Underlying Cause of Death 1999–2018 on CDC WONDER Online Database. United States, Centers for Disease Control and Prevention, http://wonder.cdc.gov/ucd-icd10.html. Accessed 10/14/2020.
-
- Harris LM, Faggioli GL, Fiedler R, Curl GR, and Ricotta JJ (1991) Ruptured abdominal aortic aneurysms: factors affecting mortality rates. Journal of vascular surgery 14, 812–818; discussion 819–820 - PubMed
-
- Shimizu K, Mitchell RN, and Libby P (2006) Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol 26, 987–994 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

