Covid-19 Breakthrough Infections in Vaccinated Health Care Workers
- PMID: 34320281
- PMCID: PMC8362591
- DOI: 10.1056/NEJMoa2109072
Covid-19 Breakthrough Infections in Vaccinated Health Care Workers
Abstract
Background: Despite the high efficacy of the BNT162b2 messenger RNA vaccine against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), rare breakthrough infections have been reported, including infections among health care workers. Data are needed to characterize these infections and define correlates of breakthrough and infectivity.
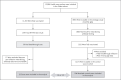
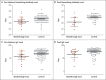
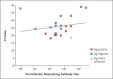
Methods: At the largest medical center in Israel, we identified breakthrough infections by performing extensive evaluations of health care workers who were symptomatic (including mild symptoms) or had known infection exposure. These evaluations included epidemiologic investigations, repeat reverse-transcriptase-polymerase-chain-reaction (RT-PCR) assays, antigen-detecting rapid diagnostic testing (Ag-RDT), serologic assays, and genomic sequencing. Correlates of breakthrough infection were assessed in a case-control analysis. We matched patients with breakthrough infection who had antibody titers obtained within a week before SARS-CoV-2 detection (peri-infection period) with four to five uninfected controls and used generalized estimating equations to predict the geometric mean titers among cases and controls and the ratio between the titers in the two groups. We also assessed the correlation between neutralizing antibody titers and N gene cycle threshold (Ct) values with respect to infectivity.
Results: Among 1497 fully vaccinated health care workers for whom RT-PCR data were available, 39 SARS-CoV-2 breakthrough infections were documented. Neutralizing antibody titers in case patients during the peri-infection period were lower than those in matched uninfected controls (case-to-control ratio, 0.361; 95% confidence interval, 0.165 to 0.787). Higher peri-infection neutralizing antibody titers were associated with lower infectivity (higher Ct values). Most breakthrough cases were mild or asymptomatic, although 19% had persistent symptoms (>6 weeks). The B.1.1.7 (alpha) variant was found in 85% of samples tested. A total of 74% of case patients had a high viral load (Ct value, <30) at some point during their infection; however, of these patients, only 17 (59%) had a positive result on concurrent Ag-RDT. No secondary infections were documented.
Conclusions: Among fully vaccinated health care workers, the occurrence of breakthrough infections with SARS-CoV-2 was correlated with neutralizing antibody titers during the peri-infection period. Most breakthrough infections were mild or asymptomatic, although persistent symptoms did occur.
Copyright © 2021 Massachusetts Medical Society.
Figures
Comment in
-
Exploring the possible link between myocarditis and mRNA COVID-19 vaccines.Eur J Intern Med. 2021 Oct;92:28-30. doi: 10.1016/j.ejim.2021.08.018. Epub 2021 Aug 28. Eur J Intern Med. 2021. PMID: 34518081 Free PMC article. No abstract available.
-
Covid-19 Breakthrough Infections in Vaccinated Health Care Workers.N Engl J Med. 2021 Oct 21;385(17):1629-1630. doi: 10.1056/NEJMc2113497. Epub 2021 Sep 29. N Engl J Med. 2021. PMID: 34587378 No abstract available.
-
Covid-19 Infections in Vaccinated Health Care Workers.N Engl J Med. 2022 Jan 13;386(2):193. doi: 10.1056/NEJMc2117817. Epub 2022 Jan 5. N Engl J Med. 2022. PMID: 34986282 No abstract available.
References
-
- Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet 2021;397:1819-1829. - PMC - PubMed
-
- Petter E, Mor O, Zuckerman N, et al. Initial real world evidence for lower viral load of individuals who have been vaccinated by BNT162b2. February 8, 2021. (https://www.medrxiv.org/content/10.1101/2021.02.08.21251329v1). preprint.
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
