A General Organocatalytic System for Electron Donor-Acceptor Complex Photoactivation and Its Use in Radical Processes
- PMID: 34320312
- PMCID: PMC8361436
- DOI: 10.1021/jacs.1c05607
A General Organocatalytic System for Electron Donor-Acceptor Complex Photoactivation and Its Use in Radical Processes
Abstract
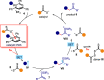
We report herein a modular class of organic catalysts that, acting as donors, can readily form photoactive electron donor-acceptor (EDA) complexes with a variety of radical precursors. Excitation with visible light generates open-shell intermediates under mild conditions, including nonstabilized carbon radicals and nitrogen-centered radicals. The modular nature of the commercially available xanthogenate and dithiocarbamate anion organocatalysts offers a versatile EDA complex catalytic platform for developing mechanistically distinct radical reactions, encompassing redox-neutral and net-reductive processes. Mechanistic investigations, by means of quantum yield determination, established that a closed catalytic cycle is operational for all of the developed radical processes, highlighting the ability of the organic catalysts to turn over and iteratively drive every catalytic cycle. We also demonstrate how the catalysts' stability and the method's high functional group tolerance could be advantageous for the direct radical functionalization of abundant functional groups, including aliphatic carboxylic acids and amines, and for applications in the late-stage elaboration of biorelevant compounds and enantioselective radical catalysis.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

















References
-
- Foster R. Electron Donor-Acceptor Complexes. J. Phys. Chem. 1980, 84, 2135–2141. 10.1021/j100454a006. - DOI
- Rosokha S. V.; Kochi J. K. Fresh Look at Electron-Transfer Mechanisms via the Donor/Acceptor Bindings in the Critical Encounter Complex. Acc. Chem. Res. 2008, 41, 641–653. 10.1021/ar700256a. - DOI - PubMed
-
- Shaw M. H.; Twilton J.; MacMillan D. W. C. Photoredox Catalysis in Organic Chemistry. J. Org. Chem. 2016, 81, 6898–6926. 10.1021/acs.joc.6b01449. - DOI - PMC - PubMed
- Wei Y.; Zhou Q.-Q.; Tan F.; Lu L.-Q.; Xiao W.-J. Visible-Light-Driven Organic Photochemical Reactions in the Absence of External Photocatalysts. Synthesis 2019, 51, 3021–3054. 10.1055/s-0037-1611812. - DOI
-
-
Selected synthetic applications of stoichiometric EDA complexes:
- Sankararaman S.; Haney W. A.; Kochi J. K. Annihilation of Aromatic Cation Radicals by Ion-Pair and Radical-Pair Collapse. Unusual Solvent and Salt Effects in the Competition for Aromatic Substitution. J. Am. Chem. Soc. 1987, 109, 7824–7838. 10.1021/ja00259a035. - DOI
- Russell G. A.; Wang K. Homolytic Alkylation of Enamines by Electrophilic Radicals. J. Org. Chem. 1991, 56, 3475–3479. 10.1021/jo00011a007. - DOI
- Tobisu M.; Furukawa T.; Chatani N. Visible Light-mediated Direct Arylation of Arenes and Heteroarenes Using Diaryliodonium Salts in the Presence and Absence of a Photocatalyst. Chem. Lett. 2013, 42, 1203–1205. 10.1246/cl.130547. - DOI
- Kandukuri S. R.; Bahamonde A.; Chatterjee I.; Jurberg I. D.; Escudero-Adán E. C.; Melchiorre P. X-Ray Characterization of an Electron Donor-Acceptor Complex Drives the Photochemical Alkylation of Indoles. Angew. Chem., Int. Ed. 2015, 54, 1485–1489. 10.1002/anie.201409529. - DOI - PubMed
- Liu B.; Lim C.-H.; Miyake G. M. Visible-Light-Promoted C–S Cross-Coupling via Intermolecular Charge Transfer. J. Am. Chem. Soc. 2017, 139, 13616–13619. 10.1021/jacs.7b07390. - DOI - PMC - PubMed
- Börgel J.; Tanwar L.; Berger F.; Ritter T. Late-Stage Aromatic C–H Oxygenation. J. Am. Chem. Soc. 2018, 140, 16026–16031. 10.1021/jacs.8b09208. - DOI - PubMed
- Xie S.; Li D.; Huang H.; Zhang F.; Chen Y. Intermolecular Radical Addition to Ketoacids Enabled by Boron Activation. J. Am. Chem. Soc. 2019, 141, 16237–16242. 10.1021/jacs.9b09099. - DOI - PubMed
- Lübbesmeyer M.; Mackay E. G.; Raycroft M. A. R.; Elfert J.; Pratt D. A.; Studer A. Base-Promoted C–C Bond Activation Enables Radical Allylation with Homoallylic Alcohols. J. Am. Chem. Soc. 2020, 142, 2609–2616. 10.1021/jacs.9b12343. - DOI - PMC - PubMed
- Kammer L. M.; Badir S. O.; Hu R.-M.; Molander G. A. Photoactive electron donor–acceptor complex platform for Ni-mediated C(sp3)–C(sp2) bond formation. Chem. Sci. 2021, 12, 5450–5457. 10.1039/D1SC00943E. - DOI - PMC - PubMed
- Liu Y.-Y.; Yu X.-Y.; Chen J.-R.; Qiao M.-M.; Qi X.; Shi D.-Q.; Xiao W.-J. Visible-Light-Driven Aza-ortho-quinone Methide Generation for the Synthesis of Indoles in a Multicomponent Reaction. Angew. Chem., Int. Ed. 2017, 56, 9527–9531. 10.1002/anie.201704690. - DOI - PubMed
-
-
-
For photochemical enzymatic processes that use cofactors as catalytic donors in EDA complexes, see the following:
- Emmanuel M. A.; Greenberg N. R.; Oblinsky D. G.; Hyster T. K. Accessing non-natural reactivity by irradiating nicotinamide-dependent enzymes with light. Nature 2016, 540, 414–417. 10.1038/nature20569. - DOI - PubMed
- Clayman P. D.; Hyster T. K. Photoenzymatic Generation of Unstabilized Alkyl Radicals: An Asymmetric Reductive Cyclization. J. Am. Chem. Soc. 2020, 142, 15673–15677. 10.1021/jacs.0c07918. - DOI - PMC - PubMed
- Page C. G.; Cooper S. J.; DeHovitz J. S.; Oblinsky D. G.; Biegasiewicz K. F.; Antropow A. H.; Armbrust K. W.; Ellis J. M.; Hamann L. G.; Horn E. J.; Oberg K. M.; Scholes G. D.; Hyster T. K. Quaternary Charge-Transfer Complex Enables Photoenzymatic Intermolecular Hydroalkylation of Olefins. J. Am. Chem. Soc. 2021, 143, 97–102. 10.1021/jacs.0c11462. - DOI - PMC - PubMed
-
For a distinct EDA complex catalytic approach, see the following:
- Quint V.; Morlet-Savary F.; Lohier J.-F.; Lalevée J.; Gaumont A.-C.; Lakhdar S. Metal-Free, Visible Light-Photocatalyzed Synthesis of Benzo[b]phosphole Oxides: Synthetic and Mechanistic Investigations. J. Am. Chem. Soc. 2016, 138, 7436–7441. 10.1021/jacs.6b04069. - DOI - PubMed
-
Publication types
LinkOut - more resources
Full Text Sources

