Resident cardiac macrophages mediate adaptive myocardial remodeling
- PMID: 34320366
- PMCID: PMC8446343
- DOI: 10.1016/j.immuni.2021.07.003
Resident cardiac macrophages mediate adaptive myocardial remodeling
Abstract
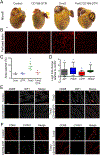
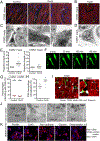
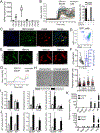
Cardiac macrophages represent a heterogeneous cell population with distinct origins, dynamics, and functions. Recent studies have revealed that C-C Chemokine Receptor 2 positive (CCR2+) macrophages derived from infiltrating monocytes regulate myocardial inflammation and heart failure pathogenesis. Comparatively little is known about the functions of tissue resident (CCR2-) macrophages. Herein, we identified an essential role for CCR2- macrophages in the chronically failing heart. Depletion of CCR2- macrophages in mice with dilated cardiomyopathy accelerated mortality and impaired ventricular remodeling and coronary angiogenesis, adaptive changes necessary to maintain cardiac output in the setting of reduced cardiac contractility. Mechanistically, CCR2- macrophages interacted with neighboring cardiomyocytes via focal adhesion complexes and were activated in response to mechanical stretch through a transient receptor potential vanilloid 4 (TRPV4)-dependent pathway that controlled growth factor expression. These findings establish a role for tissue-resident macrophages in adaptive cardiac remodeling and implicate mechanical sensing in cardiac macrophage activation.
Keywords: C-C chemokine receptor 2; CCR2; TRPV4; angiogenesis; cardiac remodeling; dilated cardiomyopathy; heart failure; macrophages; transient receptor potentialvanilloid 4.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors have no financial or competing interests to disclose.
Figures




Comment in
-
In matters of the heart, (cellular) communication is key.Immunity. 2021 Sep 14;54(9):1906-1908. doi: 10.1016/j.immuni.2021.08.004. Immunity. 2021. PMID: 34525333
References
-
- Bain CC, Scott CL, Uronen-Hansson H, Gudjonsson S, Jansson O, Grip O, Guilliams M, Malissen B, Agace WW, and Mowat AM (2013). Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol 6, 498–510. - PMC - PubMed
-
- Bajpai G, Bredemeyer A, Li W, Zaitsev K, Koenig AL, Lokshina I, Mohan J, Ivey B, Hsiao HM, Weinheimer C, et al. (2019). Tissue Resident CCR2− and CCR2+ Cardiac Macrophages Differentially Orchestrate Monocyte Recruitment and Fate Specification Following Myocardial Injury. Circ Res 124, 263–278. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL151685/HL/NHLBI NIH HHS/United States
- P41 EB025815/EB/NIBIB NIH HHS/United States
- R01 AA027065/AA/NIAAA NIH HHS/United States
- R01 HL131908/HL/NHLBI NIH HHS/United States
- R01 HL151078/HL/NHLBI NIH HHS/United States
- T32 HL125241/HL/NHLBI NIH HHS/United States
- R01 HL094601/HL/NHLBI NIH HHS/United States
- R01 HL141086/HL/NHLBI NIH HHS/United States
- R01 HL138466/HL/NHLBI NIH HHS/United States
- R21 AI148877/AI/NIAID NIH HHS/United States
- R35 HL145212/HL/NHLBI NIH HHS/United States
- R01 HL139714/HL/NHLBI NIH HHS/United States
- P01 AI116501/AI/NIAID NIH HHS/United States
- R01 DK103901/DK/NIDDK NIH HHS/United States
- S10 OD021694/OD/NIH HHS/United States
- P30 DK052574/DK/NIDDK NIH HHS/United States
- S10 OD028597/OD/NIH HHS/United States
- R01 AR077183/AR/NIAMS NIH HHS/United States
- I01 BX002730/BX/BLRD VA/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

