Evaluation of Remote Check: A Clinical Tool for Asynchronous Monitoring and Triage of Cochlear Implant Recipients
- PMID: 34320523
- PMCID: PMC8862779
- DOI: 10.1097/AUD.0000000000001106
Evaluation of Remote Check: A Clinical Tool for Asynchronous Monitoring and Triage of Cochlear Implant Recipients
Abstract
Background: A new Remote Check App permits remote self-testing of hearing function for Nucleus cochlear implant (CI) recipients and enables asynchronous review by their clinician to support patient-management decisions.
Objectives: To evaluate the Remote Check App for: (1) ease of use; (2) overall acceptance of the test battery by CI recipient or their carer in the home setting; (3) test-retest reliability of audiological threshold and speech recognition measures via wireless streaming; and (4) to compare outcomes from patient-driven measures with conventional clinician-driven measurements of aided-hearing function.
Design: Single-site, prospective, repeated-measures cohort study with 32 experienced CI users (28 adults and 4 children).
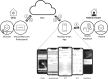
Methods: Participants completed self-testing using the Remote Check app at home and in the clinic. Measures include audiological, objective and subjective tests. Self-administered speech recognition in noise, via the digit triplets test (DTT) and aided thresholds, via the aided threshold test (ATT) were reassessed in free-field and by clinicians following conventional clinical protocols. Results of ATT and DTT were compared across test conditions. Completion time and perceived ease of self-driven assessments were documented. Insights from subsequent real-world experience with Remote Check are summarized and compared to the study findings.
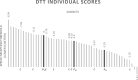
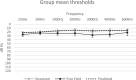
Results: Remote Check was rated as easy to use by the majority (87%) of subjects. Mean group test-retest score differences for self-administered testing within the clinic versus at-home environments were nonsignificant (p > 0.05): 1.4 dB (SD = 1.97) for ATT and 1.6 dB (SD = 1.54) for DTT. Mean group test-retest score difference for patient-driven DTT in streamed versus the free-field condition was 1.8 dB (SD = 2.02). Self-administered, streamed, ATT via Remote Check, resulted in significantly lower thresholds compared to clinician-driven warble-tone thresholds in the free-field by 6.7 dB (SD = 6.8) (p < 0.001). ATT thresholds via Remote Check were not significantly different from predicted thresholds based on the Threshold Sound Pressure Level of the sound processor.
Conclusion: Remote Check is the first CI telehealth assessment tool that uses wireless streaming to enable comprehensive, easy and reliable self-testing of hearing function by the CI recipient or their carer, in the comfort of their home. Asynchronous access to test results can assist clinicians in monitoring and triaging individuals for appropriate patient-management based on their needs. Use of remote monitoring may also help to reduce the burden of unnecessary clinic visits on clinic resources, patient travel time and associated costs. Remote Check is an important step toward addressing the current growing need for asynchronous audiological telepractice to support long-term care of CI recipients.
Copyright © 2021 The Authors. Ear & Hearing is published on behalf of the American Auditory Society, by Wolters Kluwer Health, Inc.
Figures
Similar articles
-
Personal experience with the remote check telehealth in cochlear implant users: from COVID-19 emergency to routine service.Eur Arch Otorhinolaryngol. 2023 Dec;280(12):5293-5298. doi: 10.1007/s00405-023-08045-2. Epub 2023 Jul 1. Eur Arch Otorhinolaryngol. 2023. PMID: 37393199 Free PMC article.
-
Remote Cochlear Implant Assessments: Validity and Stability in Self-Administered Smartphone-Based Testing.Ear Hear. 2024 Jan-Feb 01;45(1):239-249. doi: 10.1097/AUD.0000000000001422. Epub 2023 Aug 29. Ear Hear. 2024. PMID: 37641179
-
Remote check test battery for cochlear implant recipients: proof of concept study.Int J Audiol. 2022 Jun;61(6):443-452. doi: 10.1080/14992027.2021.1922767. Epub 2021 Aug 25. Int J Audiol. 2022. PMID: 34431430
-
Implantable Devices for Single-Sided Deafness and Conductive or Mixed Hearing Loss: A Health Technology Assessment.Ont Health Technol Assess Ser. 2020 Mar 6;20(1):1-165. eCollection 2020. Ont Health Technol Assess Ser. 2020. PMID: 32194878 Free PMC article.
-
Identification of Pure-Tone Audiologic Thresholds for Pediatric Cochlear Implant Candidacy: A Systematic Review.JAMA Otolaryngol Head Neck Surg. 2018 Jul 1;144(7):630-638. doi: 10.1001/jamaoto.2018.0652. JAMA Otolaryngol Head Neck Surg. 2018. PMID: 29800000
Cited by
-
[Empowering patients through app-based cochlear implant self-adjustment].HNO. 2025 Feb;73(2):83-94. doi: 10.1007/s00106-024-01544-6. Epub 2025 Jan 9. HNO. 2025. PMID: 39789268 Free PMC article. Review. German.
-
Digital Therapeutics in Hearing Healthcare: Evidence-Based Review.J Audiol Otol. 2024 Jul;28(3):159-166. doi: 10.7874/jao.2023.00780. Epub 2024 Jul 9. J Audiol Otol. 2024. PMID: 38973323 Free PMC article.
-
Impact of the COVID-19 pandemic on paediatric otolaryngology: a nationwide study.Acta Otorhinolaryngol Ital. 2023 Oct;43(5):352-359. doi: 10.14639/0392-100X-N2452. Epub 2023 Jul 28. Acta Otorhinolaryngol Ital. 2023. PMID: 37519138 Free PMC article.
-
Personal experience with the remote check telehealth in cochlear implant users: from COVID-19 emergency to routine service.Eur Arch Otorhinolaryngol. 2023 Dec;280(12):5293-5298. doi: 10.1007/s00405-023-08045-2. Epub 2023 Jul 1. Eur Arch Otorhinolaryngol. 2023. PMID: 37393199 Free PMC article.
-
Patient preferences for Remote cochlear implant management: A discrete choice experiment.PLoS One. 2025 Jun 3;20(6):e0320421. doi: 10.1371/journal.pone.0320421. eCollection 2025. PLoS One. 2025. PMID: 40460110 Free PMC article.
References
-
- American Academy of Audiology. Cochlear implant practice guidelines. (2019). https://www.audiology.org/sites/default/files/publications/resources/Coc.... - PubMed
-
- American National Standards Institute (ANSI). Maximum Permissible Ambient Noise Levels For Audiometric Test Rooms (ANSI/ASA S3.1-R2018). (2018). https://webstore.ansi.org/standards/asa/ansiasas31999r2018
-
- Archbold S. M., Nikolopoulos T. P., Lloyd-Richmond H. Long-term use of cochlear implant systems in paediatric recipients and factors contributing to non-use. Cochlear Implants Int, (2009). 10, 25–40. - PubMed
-
- American Speech-Language-Hearing Association (ASHA). Guidelines for Manual Pure-Tone Threshold Audiometry. (2005). https://www.asha.org/policy/gl2005-00014/
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

