Intratumoral administration of astatine-211-labeled gold nanoparticle for alpha therapy
- PMID: 34320997
- PMCID: PMC8317303
- DOI: 10.1186/s12951-021-00963-9
Intratumoral administration of astatine-211-labeled gold nanoparticle for alpha therapy
Abstract
Background: 211At is a high-energy α-ray emitter with a relatively short half-life and a high cytotoxicity for cancer cells. Its dispersion can be imaged using clinical scanners, and it can be produced in cyclotrons without the use of nuclear fuel material. This study investigated the biodistribution and the antitumor effect of 211At-labeled gold nanoparticles (211At-AuNP) administered intratumorally.
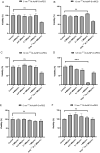
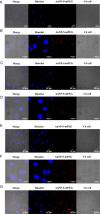
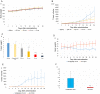
Results: AuNP with a diameter of 5, 13, 30, or 120 nm that had been modified with poly (ethylene glycol) methyl ether (mPEG) thiol and labeled with 211At (211At-AuNP-S-mPEG) were incubated with tumor cells, or intratumorally administered to C6 glioma or PANC-1 pancreatic cancers subcutaneously transplanted into rodent models. Systemic and intratumoral distributions of the particles in the rodents were then evaluated using scintigraphy and autoradiography, and the changes in tumor volumes were followed for about 40 days. 211At-AuNP-S-mPEG was cytotoxic when it was internalized by the tumor cells. After intratumoral administration, 211At-AuNP-S-mPEG became localized in the tumor and did not spread to systemic organs during a time period equivalent to 6 half-lives of 211At. Tumor growth was strongly suppressed for both C6 and PANC-1 by 211At-AuNP-S-mPEG. In the C6 glioma model, the strongest antitumor effect was observed in the group treated with 211At-AuNP-S-mPEG with a diameter of 5 nm.
Conclusions: The intratumoral single administration of a simple nanoparticle, 211At-AuNP-S-mPEG, was shown to suppress the growth of tumor tissue strongly in a particle size-dependent manner without radiation exposure to other organs caused by systemic spread of the radionuclide.
Keywords: Alpha emitters; Astatine-211; Cancer therapy; Gold nanoparticles; Radiolabeling.
© 2021. The Author(s).
Conflict of interest statement
The author(s) have no potential competing of interest to declare with respect to the conduct of the research, the authorship, and/or the publication of this article.
Figures


References
-
- Smith GD, Pickles T, Crook J, Martin AG, Vigneault E, Cury FL, Morris J, Catton C, Lukka H, Warner A, et al. Brachytherapy improves biochemical failure-free survival in low- and intermediate-risk prostate cancer compared with conventionally fractionated external beam radiation therapy: a propensity score matched analysis. Int J Radiat Oncol Biol Phys. 2015;91:505–516. doi: 10.1016/j.ijrobp.2014.11.018. - DOI - PubMed
-
- Roddiger SJ, Kolotas C, Filipowicz I, Kurek R, Kuner RP, Martin T, Baltas D, Rogge B, Kontova M, Hoffmann G, et al. Neoadjuvant interstitial high-dose-rate (HDR) brachytherapy combined with systemic chemotherapy in patients with breast cancer. Strahlenther Onkol. 2006;182:22–29. doi: 10.1007/s00066-006-1454-7. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

