Risk of herpes zoster (shingles) in patients with rheumatoid arthritis under biologic, targeted synthetic and conventional synthetic DMARD treatment: data from the German RABBIT register
- PMID: 34321218
- PMCID: PMC8762036
- DOI: 10.1136/annrheumdis-2021-220651
Risk of herpes zoster (shingles) in patients with rheumatoid arthritis under biologic, targeted synthetic and conventional synthetic DMARD treatment: data from the German RABBIT register
Abstract
Objective: To compare event and incidence rates of herpes zoster (HZ), also known as shingles, in patients with rheumatoid arthritis under treatment with conventional synthetic (cs), targeted synthetic (ts) or biologic (b) disease-modifying antirheumatic drugs (DMARDs).
Methods: Patients were prospectively enrolled from 2007 until October 2020. Reported HZ events were assigned to ongoing treatments or those terminated within 1 month prior to the HZ event. Exposure-adjusted event rates (EAERs) of HZ were calculated per 1000 patient years (py) and adjusted HRs with 95% CIs computed. Inverse probability weights (IPW) were used to adjust for confounding by indication.
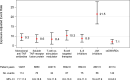
Results: Data of 13 991 patients (62 958 py) were analysed, with 559 HZ events reported in 533 patients. The EAER of HZ was highest for tsDMARDs (21.5, 95% CI 16.4 to 27.9), followed by B cell targeted therapy (10.3, 95% CI 8.0 to 13.0), monoclonal antitumour necrosis factor (anti-TNF) antibodies (9.3, 95% CI 7.7 to 11.2), interleukin 6 inhibitors (8.8, 95% CI 6.9 to 11.0), soluble TNF receptor fusion protein (8.6, 95% CI 6.8 to 10.8), T cell costimulation modulator (8.4, 95% CI 5.9 to 11.8) and csDMARDs (7.1, 95% CI 6.0 to 8.3). Adjusted for age, sex and glucocorticoids and weighted with IPW, tsDMARDs (HR 3.66, 95% CI 2.38 to 5.63), monoclonal anti-TNF antibodies (HR 1.63, 95% CI 1.17 to 2.28) and B cell targeted therapy (HR 1.57, 95% CI 1.03 to 2.40) showed a significantly higher risk compared with csDMARDs.
Conclusion: Our results provide evidence for a 3.6-fold increased risk of HZ associated with tsDMARDs and an increased risk of HZ under bDMARDs compared with csDMARDs.
Keywords: DMARDs; arthritis; biological therapy; rheumatoid; tumour necrosis factor inhibitors.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: AS has received speaking fees from Bristol-Myers Squibb, Celltrion, MSD, Pfizer and Roche. AZ has received speaking fees from AbbVie, Janssen, Pfizer, Roche and Sanofi-Aventis. G-RRB has received honoraria for lectures and consulting from AbbVie, BMS, Galapagos, Lilly, MSD, Pfizer, Roche and Sanofi. JB has received honoraria for talks, advisory boards, paid consultancies and grants for studies from AbbVie (Abbott), Amgen, Baxter, Biogen, BMS, Boehringer, Celgene, Celltrion, Centocor, Chugai, Fresenius, GlaxoSmithKline, Gilead, Hexal, Janssen, Lilly, Medac, MSD (Schering-Plough), Mylan, Mundipharma, Novartis, Pfizer (Wyeth, Hospira), Roche, Sanofi-Aventis and UCB.
Figures

References
-
- Furer V, Rondaan C, Heijstek M, et al. . Incidence and prevalence of vaccine preventable infections in adult patients with autoimmune inflammatory rheumatic diseases (AIIRD): a systemic literature review Informing the 2019 update of the EULAR recommendations for vaccination in adult patients with AIIRD. RMD Open 2019;5:e001041. 10.1136/rmdopen-2019-001041 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

