Blockade of the CD93 pathway normalizes tumor vasculature to facilitate drug delivery and immunotherapy
- PMID: 34321321
- PMCID: PMC8749958
- DOI: 10.1126/scitranslmed.abc8922
Blockade of the CD93 pathway normalizes tumor vasculature to facilitate drug delivery and immunotherapy
Abstract
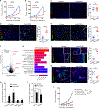
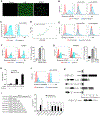
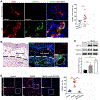
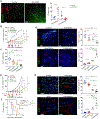
The immature and dysfunctional vascular network within solid tumors poses a substantial obstacle to immunotherapy because it creates a hypoxic tumor microenvironment that actively limits immune cell infiltration. The molecular basis underpinning this vascular dysfunction is not fully understood. Using genome-scale receptor array technology, we showed here that insulin-like growth factor binding protein 7 (IGFBP7) interacts with its receptor CD93, and we subsequently demonstrated that this interaction contributes to abnormal tumor vasculature. Both CD93 and IGFBP7 were up-regulated in tumor-associated endothelial cells. IGFBP7 interacted with CD93 via a domain different from multimerin-2, the known ligand for CD93. In two mouse tumor models, blockade of the CD93/IGFBP7 interaction by monoclonal antibodies promoted vascular maturation to reduce leakage, leading to reduced tumor hypoxia and increased tumor perfusion. CD93 blockade in mice increased drug delivery, resulting in an improved antitumor response to gemcitabine or fluorouracil. Blockade of the CD93 pathway triggered a substantial increase in intratumoral effector T cells, thereby sensitizing mouse tumors to immune checkpoint therapy. Last, analysis of samples from patients with cancer under anti-programmed death 1/programmed death-ligand 1 treatment revealed that overexpression of the IGFBP7/CD93 pathway was associated with poor response to therapy. Thus, our study identified a molecular interaction involved in tumor vascular dysfunction and revealed an approach to promote a favorable tumor microenvironment for therapeutic intervention.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures

References
-
- De Palma M, Biziato D, Petrova TV, Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer 17, 457–474 (2017). - PubMed
-
- Carmeliet P, Jain RK, Angiogenesis in cancer and other diseases. Nature 407, 249–257 (2000). - PubMed
-
- McDonald DM, Baluk P, Significance of blood vessel leakiness in cancer. Cancer Res 62, 5381–5385 (2002). - PubMed
-
- Dreher MR, Liu W, Michelich CR, Dewhirst MW, Yuan F, Chilkoti A, Tumor vascular permeability, accumulation, and penetration of macromolecular drug carriers. J. Natl. Cancer Inst 98, 335–344 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

