Evaluation of Five International HBV Treatment Guidelines: Recommendation for Resource-Limited Developing Countries Based on the National Study in Nepal
- PMID: 34321716
- PMCID: PMC8315108
- DOI: 10.3390/pathophysiology27010002
Evaluation of Five International HBV Treatment Guidelines: Recommendation for Resource-Limited Developing Countries Based on the National Study in Nepal
Abstract
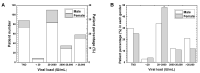
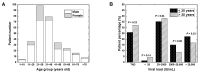
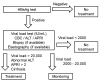
Hepatitis B virus (HBV) infects the liver, causing cirrhosis and cancer. In developed countries, five international guidelines have been used to make a decision for the management of patients with chronic HBV infection. In this review, since the guidelines were established by clinical and epidemiological data of developed countries, we aimed to evaluate whether (1) HBV patient profiles of developing countries are similar to developed countries, and (2) which guideline can be applicable to resource-limited developing countries. First, as an example of the most recent data of HBV infections among developing countries, we evaluated the national HBV viral load study in Nepal, which were compared with the data from other developing countries. In Nepal, the highest number of patients had viral loads of 20-2000 IU/mL (36.7%) and belonged to the age group of 21-30 years; HBV epidemiology in Nepal, based on the viral loads, gender, and age groups was similar to those of not only other developing countries but also developed countries. Next, we reviewed five international HBV treatment guidelines of the World Health Organization (WHO), American Association for the Study of Liver Diseases (AASLD), National Institute for Health and Care Excellence (NICE), European Association for the Study of the Liver (EASL), and Asian Pacific Association for the Study of the Liver (APASL). All guidelines require the viral load and alanine aminotransferase (ALT) levels for decision making. Although four guidelines recommend elastography to assess liver cirrhosis, the WHO guideline alternatively recommends using the aspartate aminotransferase (AST)-to-platelet ratio index (APRI), which is inexpensive and conducted routinely in most hospitals. Therefore, in resource-limited developing countries like Nepal, we recommend the WHO guideline for HBV treatment based on the viral load, ALT, and APRI information.
Keywords: DNA viruses; Hepadnaviridiae; chronic human hepatitis; elastography; viral diseases.
Conflict of interest statement
Conflicts of Interest: The authors declare no conflict of interest.
Figures
Similar articles
-
Accuracy of international guidelines for identifying significant fibrosis in hepatitis B e antigen--negative patients with chronic hepatitis.Clin Gastroenterol Hepatol. 2013 Nov;11(11):1493-1499.e2. doi: 10.1016/j.cgh.2013.05.038. Epub 2013 Jun 28. Clin Gastroenterol Hepatol. 2013. PMID: 23811251
-
HBV DNA level could predict significant liver fibrosis in HBeAg negative chronic hepatitis B patients with biopsy indication.BMC Gastroenterol. 2014 Dec 19;14:218. doi: 10.1186/s12876-014-0218-6. BMC Gastroenterol. 2014. PMID: 25523185 Free PMC article.
-
The 2024 updated WHO guidelines for the prevention and management of chronic hepatitis B: Main changes and potential implications for the next major liver society clinical practice guidelines.J Hepatol. 2025 May;82(5):918-925. doi: 10.1016/j.jhep.2024.12.004. Epub 2024 Dec 6. J Hepatol. 2025. PMID: 39647534 Review.
-
Liver fibrosis progression is uncommon in patients with inactive chronic hepatitis B: a prospective cohort study with paired transient elastography examination.J Gastroenterol Hepatol. 2013 Dec;28(12):1842-8. doi: 10.1111/jgh.12327. J Gastroenterol Hepatol. 2013. PMID: 23829381
-
Treatment of chronic hepatitis B: case selection and duration of therapy.J Gastroenterol Hepatol. 2002 Apr;17(4):409-14. doi: 10.1046/j.1440-1746.2002.02767.x. J Gastroenterol Hepatol. 2002. PMID: 11982721 Review.
Cited by
-
An Optimized Strategy Based on Conventional Ultrasound for Diagnosing Metabolic Dysfunction-Associated Steatotic Liver Disease.Diagnostics (Basel). 2023 Nov 22;13(23):3503. doi: 10.3390/diagnostics13233503. Diagnostics (Basel). 2023. PMID: 38066744 Free PMC article.
-
Geospatial patterns and socioeconomic determinants of the global acute viral hepatitis burden.Front Public Health. 2025 Jun 5;13:1581484. doi: 10.3389/fpubh.2025.1581484. eCollection 2025. Front Public Health. 2025. PMID: 40538691 Free PMC article.
-
Role of Ultrasound Methods for the Assessment of NAFLD.J Clin Med. 2022 Aug 5;11(15):4581. doi: 10.3390/jcm11154581. J Clin Med. 2022. PMID: 35956196 Free PMC article. Review.
References
-
- William F., Nauschuetz S.L.L. Clinical virology. In: Connie R., Mahon D.C.L., Manuselis G., editors. Textbook of Diagnostics Microbiology. 5th ed. Elsevier; Missouri, MO, USA: 2015. pp. 688–726.
-
- WHO . Guideline for the Prevention Care and Treatment of Persons with Chronic Hepatitis B Infection. World Health Organization; Geneva, Switzerland: 2015. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
