Antibacterial Effect of Honey-Derived Exosomes Containing Antimicrobial Peptides Against Oral Streptococci
- PMID: 34321877
- PMCID: PMC8312616
- DOI: 10.2147/IJN.S315040
Antibacterial Effect of Honey-Derived Exosomes Containing Antimicrobial Peptides Against Oral Streptococci
Abstract
Purpose: Recently, our group found exosome-like extracellular vesicles (EVs) in Apis mellifera honey displaying strong antibacterial effects; however, the underlying mechanism is still not understood. Thus, the aim of this investigation was to characterize the molecular and nanomechanical properties of A. mellifera honey-derived EVs in order to elucidate the mechanisms behind their antibacterial effect, as well as to determine differential antibiofilm properties against relevant oral streptococci.
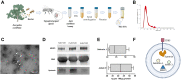
Methods: A. mellifera honey-derived EVs (HEc-EVs) isolated via ultracentrifugation were characterized with Western Blot and ELISA to determine the presence of specific exosomal markers and antibacterial cargo, and atomic force microscopy (AFM) was utilized to explore their ultrastructural and nanomechanical properties via non-destructive immobilization onto poly-L-lysine substrates. Furthermore, the effect of HEc-EVs on growth and biofilm inhibition of S. mutans was explored with microplate assays and compared to S. sanguinis. AFM was utilized to describe ultrastructural and nanomechanical alterations such as cell wall elasticity changes following HEc-EV exposure.
Results: Molecular characterization of HEc-EVs identified for the first time important conserved exosome markers such as CD63 and syntenin, and the antibacterial molecules MRJP1, defensin-1 and jellein-3 were found as intravesicular cargo. Nanomechanical characterization revealed that honey-derived EVs were mostly <150nm, with elastic modulus values in the low MPa range, comparable to EVs from other biological sources. Furthermore, incubating oral streptococci with EVs confirmed their antibacterial and antibiofilm capacities, displaying an increased effect on S. mutans compared to S. sanguinis. AFM nanocharacterization showed topographical and nanomechanical alterations consistent with membrane damage on S. mutans.
Conclusion: Honey is a promising new source of highly active EVs with exosomal origin, containing a number of antibacterial peptides as cargo molecules. Furthermore, the differential effect of HEC-EVs on S. mutans and S. sanguinis may serve as a novel biofilm-modulating strategy in dental caries.
Keywords: atomic force microscopy; biofilms; dental caries; honey.
© 2021 Leiva-Sabadini et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures



Similar articles
-
Antibacterial and Antibiofilm Effect of Honey in the Prevention of Dental Caries: A Recent Perspective.Foods. 2022 Sep 2;11(17):2670. doi: 10.3390/foods11172670. Foods. 2022. PMID: 36076855 Free PMC article. Review.
-
Effects of Antimicrobial Peptide GH12 on the Cariogenic Properties and Composition of a Cariogenic Multispecies Biofilm.Appl Environ Microbiol. 2018 Nov 30;84(24):e01423-18. doi: 10.1128/AEM.01423-18. Print 2018 Dec 15. Appl Environ Microbiol. 2018. PMID: 30341079 Free PMC article.
-
Nanomechanical characterization of exosomes and concomitant nanoparticles from blood plasma by PeakForce AFM in liquid.Biochim Biophys Acta Gen Subj. 2022 Jul;1866(7):130139. doi: 10.1016/j.bbagen.2022.130139. Epub 2022 Apr 4. Biochim Biophys Acta Gen Subj. 2022. PMID: 35390487
-
Selective antibacterial activity of a novel lactotransferrin-derived antimicrobial peptide LF-1 against Streptococcus mutans.Arch Oral Biol. 2022 Jul;139:105446. doi: 10.1016/j.archoralbio.2022.105446. Epub 2022 Apr 26. Arch Oral Biol. 2022. PMID: 35512618
-
Nanocharacterization in dentistry.Int J Mol Sci. 2010 Jun 17;11(6):2523-45. doi: 10.3390/ijms11062523. Int J Mol Sci. 2010. PMID: 20640166 Free PMC article. Review.
Cited by
-
Antibacterial and Antibiofilm Effect of Honey in the Prevention of Dental Caries: A Recent Perspective.Foods. 2022 Sep 2;11(17):2670. doi: 10.3390/foods11172670. Foods. 2022. PMID: 36076855 Free PMC article. Review.
-
Cannabidiol-Loaded Extracellular Vesicles from Human Umbilical Cord Mesenchymal Stem Cells Alleviate Paclitaxel-Induced Peripheral Neuropathy.Pharmaceutics. 2023 Feb 7;15(2):554. doi: 10.3390/pharmaceutics15020554. Pharmaceutics. 2023. PMID: 36839877 Free PMC article.
-
Bacterial extracellular vesicle applications in cancer immunotherapy.Bioact Mater. 2022 Oct 31;22:551-566. doi: 10.1016/j.bioactmat.2022.10.024. eCollection 2023 Apr. Bioact Mater. 2022. PMID: 36382022 Free PMC article.
-
Harnessing Nature's Defence: The Antimicrobial Efficacy of Pasteurised Cattle Milk-Derived Extracellular Vesicles on Staphylococcus aureus ATCC 25923.Int J Mol Sci. 2024 Apr 26;25(9):4759. doi: 10.3390/ijms25094759. Int J Mol Sci. 2024. PMID: 38731976 Free PMC article.
-
Bioinspired Nanoplatforms: Polydopamine and Exosomes for Targeted Antimicrobial Therapy.Polymers (Basel). 2025 Jun 16;17(12):1670. doi: 10.3390/polym17121670. Polymers (Basel). 2025. PMID: 40574198 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

