Obinutuzumab Plus Chemotherapy Compared with Rituximab Plus Chemotherapy in Previously Untreated Italian Patients with Advanced Follicular Lymphoma at Intermediate-High Risk: A Cost-Effectiveness Analysis
- PMID: 34321898
- PMCID: PMC8313400
- DOI: 10.2147/CEOR.S317885
Obinutuzumab Plus Chemotherapy Compared with Rituximab Plus Chemotherapy in Previously Untreated Italian Patients with Advanced Follicular Lymphoma at Intermediate-High Risk: A Cost-Effectiveness Analysis
Abstract
Objective: To assess the cost-effectiveness of obinutuzumab (O-chemo) in comparison to rituximab (R-chemo) in patients with untreated advanced follicular lymphoma (FL) at intermediate or high risk from an Italian National Health Service (NHS) perspective.
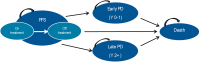
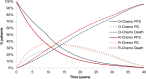
Methods: A previously developed four-state Markov model was adapted to estimate lifetime clinical outcomes and costs of Italian patients with advanced FL and an FL international predictive index score ≥2 in treatment with O-chemo and R-chemo. Life expectancy was derived from the GALLIUM and PRIMA clinical trials. Progression-free survival (PFS), early progressive disease (PD), and treatment duration were extrapolated by fitting parametric distributions to empirical data in GALLIUM and late PD to data in PRIMA. Expected survival was weighed by published utilities. Costs updated to 2020 Euros and health gains occurring after the first year were discounted at an annual 3% rate. Probabilistic sensitivity analysis (PSA) was carried out.
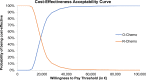
Results: O-chemo was associated with an incremental survival increase (0.97 life-years [LYs]), even when weighted for quality (0.88 quality-adjusted LYs [QALYs]), and incremental costs (around €15,000), driven by longer treatment during PFS state relative to R-chemo. The incremental cost-effectiveness ratio and incremental cost-utility ratio are both widely accepted by the Italian NHS (around €15,500/LY and €17,000/QALY gained, respectively). PSA simulations confirmed the robustness of results given sensible variations in assumptions.
Conclusion: O-chemo has superior clinical efficacy compared to rituximab, and should be considered a cost-effective option in first-line treatment of patients with advanced FL at intermediate or high risk in Italy. Incremental cost-effectiveness ratios are below the threshold considered affordable by developed countries.
Keywords: FLIPI score; ICER; PFS; QALY; economic evaluation; oncology.
© 2021 Bellone et al.
Conflict of interest statement
Dr Marco Bellone is an employee of AdRes, which has received project funding from Roche for the development of this research. Dr Marco Bellone reports grants from Roche during the study and grants from Roche, Sanofi, Janssen-Cilag Spa, Fresenius Kabi Deutschland, Novartis Farma, and Fresenius Kabi Italia outside the submitted work. Dr Lorenzo Pradelli is co-owner and an employee of AdRes, which has received project funding from Roche for the development of this research. Dr Lorenzo Pradelli report grants from Roche Spa during the study and grants from Janssen-Cilag, Sanofi, AstraZeneca, Biogen, and Medtronic outside the submitted work. Drs Stefano Molica and Adele Emanuela De Francesco are consultants for Roche. Drs Daniela Ghislieri, Emanuele Guardalben, and Antonietta Caputo are employees of Roche. The abstract of this paper was presented at the ISPOR Europe 2019 as a poster presentation, and can be viewed here: https://doi.org/10.1016/j.jval.2019.09.352
Figures

References
-
- Al-Hamadani M, Habermann TM, Cerhan JR, Macon WR, Maurer MJ, Go RS. Non-Hodgkin lymphoma subtype distribution, geodemographic patterns, and survival in the US: a longitudinal analysis of the National Cancer Data Base from 1998 to 2011. Am J Hematol. 2015;90(9):790–795. doi:10.1002/ajh.24086 - DOI - PubMed
-
- Smith A, Crouch S, Howell D, Burton C, Patmore R, Roman E. Impact of age and socioeconomic status on treatment and survival from aggressive lymphoma: a UK population-based study of diffuse large B-cell lymphoma. Cancer Epidemiol. 2015;39(6):1103–1112. doi:10.1016/j.canep.2015.08.015 - DOI - PMC - PubMed
-
- European Medicines Agency. European Medicines Agency: public Summary of Opinion on Orphan Designation: obinutuzumab for the treatment of follicular lymphoma 23 July 2015. Available from: https://www.ema.europa.eu/en/documents/orphan-designation/eu/3/15/1504-p.... Accessed January, 2020.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

