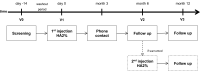
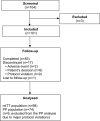
One-Year, Efficacy and Safety Open Label Study, with a Single Injection of a New Hyaluronan for Knee OA: The SOYA Trial
- PMID: 34321921
- PMCID: PMC8312328
- DOI: 10.2147/JPR.S321841
One-Year, Efficacy and Safety Open Label Study, with a Single Injection of a New Hyaluronan for Knee OA: The SOYA Trial
Abstract
Purpose: To assess the efficacy and safety of a single injection of a new formulation of hyaluronic acid (MPS-HA2%) in patients with symptomatic knee osteoarthritis after 12 months' follow-up.
Patients and methods: Prospective, single-arm, multicentre, open-label, 12-month follow-up study. Patients with Kellgren-Lawrence (KL) 2-3 and visual analogue scale (VAS) pain scores of ≥40-< 80 mm received a single injection of MPS-HA2%. The primary outcome was the reduction in VAS pain scores from baseline, and the secondary outcomes were the Western Ontario and McMaster (WOMAC) Universities Osteoarthritis Index, the minimum clinically important improvement (MCII), and patient and investigator global assessments (PGA, IGA) measured on 5-point Likert scale. Adverse events were recorded throughout the study for safety purposes.
Results: A total of 101 patients (mean age: 68 years; 74% female; and 78% overweight) were included. The mean reduction in pain at 12 months was 37.7%; the total WOMAC score improved by 36.5% and the pain, stiffness and physical function subscores returned improvements of 32.1%, 34.1% and 32.7%, respectively (p=0.0001 with respect to baseline). At 12 months, a statistically significant 62.2% of patients obtained an improvement equal to or greater than the MCII. The mean PGA score at baseline was 2.44 and 1.46 at 12 months (p<0.05), and the mean IGA scores at equivalent timepoints were 2.29 and 1.48 (p<0.05). Fourteen patients received a second injection at the 6-month follow-up visit. Eight patients reported a total of 12 treatment-related adverse events that were local, non-serious and of mild-to-moderate intensity.
Conclusion: With just a single intra-articular injection, this not controlled trial suggests that MPS-HA2% is effective 12 months after the procedure in most cases. Patient tolerability and safety were both optimal (NCT03852914).
Keywords: hyaluronic acid; osteoarthritis; pain; viscosupplementation.
© 2021 Gavín et al.
Conflict of interest statement
F. J. Blanco report grants, personal fees from Meiji Pharma Spain. P. Coronel and M. Gimeno are employees of Meiji Pharma Spain. The other authors report no conflict of interest in this work.
Figures
Similar articles
-
Comparison of Single Intra-Articular Injection of Novel Hyaluronan (HYA-JOINT Plus) with Synvisc-One for Knee Osteoarthritis: A Randomized, Controlled, Double-Blind Trial of Efficacy and Safety.J Bone Joint Surg Am. 2017 Mar 15;99(6):462-471. doi: 10.2106/JBJS.16.00469. J Bone Joint Surg Am. 2017. PMID: 28291178 Clinical Trial.
-
Comparing efficacy of intraarticular single crosslinked Hyaluronan (HYAJOINT Plus) and platelet-rich plasma (PRP) versus PRP alone for treating knee osteoarthritis.Sci Rep. 2021 Jan 8;11(1):140. doi: 10.1038/s41598-020-80333-x. Sci Rep. 2021. PMID: 33420185 Free PMC article. Clinical Trial.
-
Safety and efficacy of single CHAP Hyaluronan injection versus three injections of linear Hyaluronan in pain relief for knee osteoarthritis: a prospective, 52-week follow-up, randomized, evaluator-blinded study.BMC Musculoskelet Disord. 2021 Jun 23;22(1):572. doi: 10.1186/s12891-021-04467-3. BMC Musculoskelet Disord. 2021. PMID: 34162365 Free PMC article. Clinical Trial.
-
The Therapeutic Effect of Intra-articular Normal Saline Injections for Knee Osteoarthritis: A Meta-analysis of Evidence Level 1 Studies.Am J Sports Med. 2017 Sep;45(11):2647-2653. doi: 10.1177/0363546516680607. Epub 2016 Dec 27. Am J Sports Med. 2017. PMID: 28027657 Review.
-
Platelet-Rich Plasma Combined With Hyaluronic Acid Improves Pain and Function Compared With Hyaluronic Acid Alone in Knee Osteoarthritis: A Systematic Review and Meta-analysis.Arthroscopy. 2021 Apr;37(4):1277-1287.e1. doi: 10.1016/j.arthro.2020.11.052. Epub 2020 Dec 3. Arthroscopy. 2021. PMID: 33278533
Cited by
-
Beyond Boundaries of a Trial: Post-Market Clinical Follow-Up of SOYA Patients.J Clin Med. 2024 Oct 22;13(21):6308. doi: 10.3390/jcm13216308. J Clin Med. 2024. PMID: 39518447 Free PMC article.
-
Impact of intra-articular high-concentration hyaluronic acid administration on the innate immune response in experimental knee osteoarthritis.BMC Musculoskelet Disord. 2025 Aug 12;26(1):778. doi: 10.1186/s12891-025-09061-5. BMC Musculoskelet Disord. 2025. PMID: 40797305 Free PMC article.
-
Navigating the New EU Medical Devices Regulation: Retrospective Post-Market Follow-Up of Hyaluronic Acid Injections for Knee Osteoarthritis.Open Access Rheumatol. 2024 Mar 21;16:67-73. doi: 10.2147/OARRR.S446572. eCollection 2024. Open Access Rheumatol. 2024. PMID: 38529260 Free PMC article.
References
-
- Herrero-Beaumont G, Pérez-Baos S, Sánchez-Pernaute O, Roman-Blas JA, Lamuedra A, Largo R. Targeting chronic innate inflammatory pathways, the main road to prevention of osteoarthritis progression. Biochem Pharmacol. 2019;165:24–32. - PubMed
-
- Deveza LA, Melo L, Yamato TP, Mills K, Ravi V, Hunter DJ. Knee osteoarthritis phenotypes and their relevance for outcomes: a systematic review. Osteoarthritis Cartilage. 2017;25:1926–1941. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous