Rodent Malaria Erythrocyte Preference Assessment by an Ex Vivo Tropism Assay
- PMID: 34322397
- PMCID: PMC8311856
- DOI: 10.3389/fcimb.2021.680136
Rodent Malaria Erythrocyte Preference Assessment by an Ex Vivo Tropism Assay
Abstract
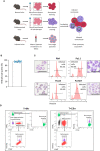
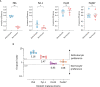
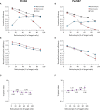
Circulating red blood cells consist of young erythrocytes (early and late reticulocytes) and mature erythrocytes (normocytes). The human malaria parasites, Plasmodium falciparum and P. vivax, have a preference to invade reticulocytes during blood-stage infection. Rodent malaria parasites that also prefer reticulocytes could be useful tools to study human malaria reticulocyte invasion. However, previous tropism studies of rodent malaria are inconsistent from one another, making it difficult to compare cell preference of different parasite species and strains. In vivo measurements of cell tropism are also subjected to many confounding factors. Here we developed an ex vivo tropism assay for rodent malaria with highly purified fractions of murine reticulocytes and normocytes. We measured invasion into the different erythrocyte populations using flow cytometry and evaluated the tropism index of the parasite strains. We found that P. berghei ANKA displayed the strongest reticulocyte preference, followed by P. yoelii 17X1.1, whereas P. chabaudi AS and P. vinckei S67 showed mixed tropism. These preferences are intrinsic and were maintained at different reticulocyte and normocyte availabilities. Our study shed light on the true erythrocyte preference of the parasites and paves the way for future investigations on the receptor-ligand interactions mediating erythrocyte tropism.
Keywords: erythrocyte invasion; erythrocyte tropism; flow cytometry; normocyte; reticulocyte; rodent malaria.
Copyright © 2021 Leong, Lee, Rénia and Malleret.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Bafort J. (1967). La Transmission Cyclique Du Plasmodium Vinckei . Ann. Soc Belg. Med. Trop. 47, 271–276. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

