Genome-Wide Analysis Identifies Rag1 and Rag2 as Novel Notch1 Transcriptional Targets in Thymocytes
- PMID: 34322489
- PMCID: PMC8311795
- DOI: 10.3389/fcell.2021.703338
Genome-Wide Analysis Identifies Rag1 and Rag2 as Novel Notch1 Transcriptional Targets in Thymocytes
Abstract
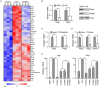
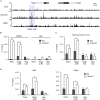
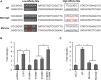
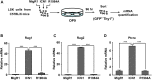
Recombination activating genes 1 (Rag1) and Rag2 are expressed in immature lymphocytes and essential for generating the vast repertoire of antigen receptors. Yet, the mechanisms governing the transcription of Rag1 and Rag2 remain to be fully determined, particularly in thymocytes. Combining cDNA microarray and ChIP-seq analysis, we identify Rag1 and Rag2 as novel Notch1 transcriptional targets in acute T-cell lymphoblastic leukemia (T-ALL) cells. We further demonstrate that Notch1 transcriptional complexes directly bind the Rag1 and Rag2 locus in not only T-ALL but also primary double negative (DN) T-cell progenitors. Specifically, dimeric Notch1 transcriptional complexes activate Rag1 and Rag2 through a novel cis-element bearing a sequence-paired site (SPS). In T-ALL and DN cells, dimerization-defective Notch1 causes compromised Rag1 and Rag2 expression; conversely, dimerization-competent Notch1 achieves optimal upregulation of both. Collectively, these results reveal Notch1 dimerization-mediated transcription as one of the mechanisms for activating Rag1 and Rag2 expression in both primary and transformed thymocytes. Our data suggest a new role of Notch1 dimerization in compelling efficient TCRβ rearrangements in DN progenitors during T-cell development.
Keywords: Double negative thymocyte; Notch1 dimerization; Recombination activating genes; T-cell acute lymphoblastic leukemia; T-cell development.
Copyright © 2021 Dong, Guo, Wang, Tu, Qing and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Reciprocal regulation of Rag expression in thymocytes by the zinc-finger proteins, Zfp608 and Zfp609.Genes Immun. 2013 Jan;14(1):7-12. doi: 10.1038/gene.2012.47. Epub 2012 Oct 18. Genes Immun. 2013. PMID: 23076336 Free PMC article.
-
Cloning and characterization of the human recombination activating gene 1 (RAG1) and RAG2 promoter regions.J Immunol. 1997 Nov 1;159(9):4382-94. J Immunol. 1997. PMID: 9379036
-
Rag1 and rag2 gene expressions identify lymphopoietic tissues in juvenile and adult Chinese giant salamander (Andrias davidianus).Dev Comp Immunol. 2018 Oct;87:24-35. doi: 10.1016/j.dci.2018.05.018. Epub 2018 May 22. Dev Comp Immunol. 2018. PMID: 29800626
-
The roles of the RAG1 and RAG2 "non-core" regions in V(D)J recombination and lymphocyte development.Arch Immunol Ther Exp (Warsz). 2009 Mar-Apr;57(2):105-16. doi: 10.1007/s00005-009-0011-3. Epub 2009 Mar 31. Arch Immunol Ther Exp (Warsz). 2009. PMID: 19333736 Review.
-
Transposition mediated by RAG1 and RAG2 and the evolution of the adaptive immune system.Immunol Res. 1999;19(2-3):169-82. doi: 10.1007/BF02786485. Immunol Res. 1999. PMID: 10493171 Review.
Cited by
-
Established and Emerging Methods for Protecting Linear DNA in Cell-Free Expression Systems.Methods Protoc. 2023 Mar 30;6(2):36. doi: 10.3390/mps6020036. Methods Protoc. 2023. PMID: 37104018 Free PMC article. Review.
-
The initial age-associated decline in early T-cell progenitors reflects fewer pre-thymic progenitors and altered signals in the bone marrow and thymus microenvironments.Aging Cell. 2023 Aug;22(8):e13870. doi: 10.1111/acel.13870. Epub 2023 May 23. Aging Cell. 2023. PMID: 37221658 Free PMC article.
-
SHIP1 Is Present but Strongly Downregulated in T-ALL, and after Restoration Suppresses Leukemia Growth in a T-ALL Xenotransplantation Mouse Model.Cells. 2023 Jul 6;12(13):1798. doi: 10.3390/cells12131798. Cells. 2023. PMID: 37443832 Free PMC article.
-
Epigenetic regulation and T-cell responses in endometriosis - something other than autoimmunity.Front Immunol. 2022 Jul 22;13:943839. doi: 10.3389/fimmu.2022.943839. eCollection 2022. Front Immunol. 2022. PMID: 35935991 Free PMC article. Review.
-
Generation and functional analysis of melanoma antigen-specific CD8+ T cells derived from S/MAR vector-transfected human induced pluripotent stem cells.Int J Cancer. 2025 Nov 1;157(9):1876-1887. doi: 10.1002/ijc.35524. Epub 2025 Jun 12. Int J Cancer. 2025. PMID: 40504044 Free PMC article.
References
-
- Ashworth T. D., Pear W. S., Chiang M. Y., Blacklow S. C., Mastio J., Xu L., et al. (2010). Deletion-based mechanisms of Notch1 activation in T-ALL: key roles for RAG recombinase and a conserved internal translational start site in Notch1. Blood 116 5455–5464. 10.1182/blood-2010-05-286328 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

