Molecular mechanisms of retinal ischemia
- PMID: 34322649
- PMCID: PMC8315104
- DOI: 10.1016/j.cophys.2018.12.008
Molecular mechanisms of retinal ischemia
Abstract
Each day, the retina converts an immense number of photons into chemical signals that are then transported to higher order neural centers for interpretation. This process of photo transduction requires large quantities of cellular energy and anabolic precursors, making the retina one of the most metabolically active tissues in the body. With such a large metabolic demand, the retina is understandably sensitive to perturbations in perfusion and hypoxia. Indeed, retinal ischemia underlies many prevalent retinal disorders including diabetic retinopathy (DR), retinal vein occlusion (RVO), and retinopathy of prematurity (ROP). Retinal ischemia leads to the expression of growth factors, cytokines, and other cellular mediators which promote inflammation, vascular dysfunction, and ultimately, vision loss. This review aims to highlight the most recent and compelling findings that have advanced our understanding of the molecular mechanisms underlying retinal ischemias.
Conflict of interest statement
Conflict of interest statement Nothing declared.
Figures

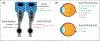
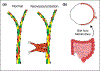
References
-
- Epstein Andrew CR, Gleadle Jonathan M, McNeill Luke A, Hewitson Kirsty S, O’Rourke John, Mole David R, Mukherji Mridul et al.: C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 2001, 107:43–54 10.1016/S0092-8674(01)00507-4. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
