Venetoclax plus low-dose cytarabine in Japanese patients with untreated acute myeloid leukaemia ineligible for intensive chemotherapy
- PMID: 34322703
- PMCID: PMC8405845
- DOI: 10.1093/jjco/hyab112
Venetoclax plus low-dose cytarabine in Japanese patients with untreated acute myeloid leukaemia ineligible for intensive chemotherapy
Abstract
Background: In a multinational phase 3 trial (VIALE-C), venetoclax plus low-dose cytarabine prolonged overall survival vs placebo plus low-dose cytarabine in patients with newly diagnosed acute myeloid leukaemia ineligible for intensive chemotherapy, although it was not statistically significant. Herein, we assess the benefit of venetoclax plus low-dose cytarabine in the Japanese subgroup of VIALE-C patients (n = 27).
Methods: VIALE-C, a randomized (2:1), double-blind study (NCT03069352), enrolled untreated patients (≥18 years) with acute myeloid leukaemia. Patients received venetoclax (600 mg days 1-28, 4-day ramp-up in cycle 1) or placebo in 28-day cycles with low-dose cytarabine (20 mg/m2 days 1-10). The primary endpoint was median overall survival.
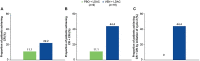
Results: In the Japanese subgroup, at a 6-month follow-up from the primary analysis, median overall survival for venetoclax (n = 18) and placebo (n = 9), plus low-dose cytarabine, was 4.7 and 8.1 months, respectively (hazard ratio, 0.928, 95% confidence intervals : 0.399, 2.156). The rate of complete remission plus complete remission with incomplete blood count recovery was higher with venetoclax plus low-dose cytarabine (44.4%) vs placebo plus low-dose cytarabine (11.1%). All patients experienced at least 1 adverse event. The most common grade ≥3 adverse events with venetoclax or placebo, plus low-dose cytarabine, were febrile neutropenia (50.0% vs 44.4%, respectively) and thrombocytopenia (27.8% vs 44.4%, respectively). Serious adverse events were reported in 50.0 and 33.3% of patients in the venetoclax and placebo, plus low-dose cytarabine arms, respectively; pneumonia was the most common (22.2% each).
Conclusions: Limited survival benefit in the Japanese subgroup can be attributed to small patient numbers and to baseline imbalances observed between treatment arms, with more patients in the venetoclax plus low-dose cytarabine arm presenting poor prognostic factors. Venetoclax plus low-dose cytarabine was well tolerated in Japanese patients with acute myeloid leukaemia ineligible for intensive chemotherapy.
Keywords: Japan; VIALE-C; acute myeloid leukaemia; low-dose cytarabine; venetoclax.
© The Author(s) 2021. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures



References
-
- Miyawaki S. Clinical studies of acute myeloid leukemia in the Japan Adult Leukemia Study Group. Int J Hematol 2012;96:171–7. - PubMed
-
- Tasaki T, Yamauchi T, Matsuda Y, et al. The response to induction therapy is crucial for the treatment outcomes of elderly patients with acute myeloid leukemia: single-institution experience. Anticancer Res 2014;34:5631–6. - PubMed
-
- Phekoo KJ, Richards MA, Møller H, Schey SA. South Thames Haematology Specialist Committee. The incidence and outcome of myeloid malignancies in 2,112 adult patients in southeast England. Haematologica 2006;91:1400–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

