Spindle cell lesions of the breast: a diagnostic approach
- PMID: 34322734
- PMCID: PMC8983634
- DOI: 10.1007/s00428-021-03162-x
Spindle cell lesions of the breast: a diagnostic approach
Abstract
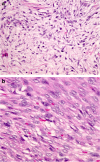
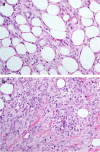
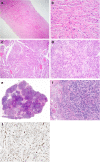
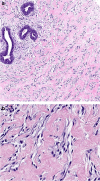
Spindle cell lesions of the breast comprise a heterogeneous group of lesions, ranging from reactive and benign processes to aggressive malignant tumours. Despite their rarity, they attract the attention of breast pathologists due to their overlapping morphological features and diagnostic challenges, particularly on core needle biopsy (CNB) specimens. Pathologists should recognise the wide range of differential diagnoses and be familiar with the diverse morphological appearances of these lesions to make an accurate diagnosis and to suggest proper management of the patients. Clinical history, immunohistochemistry, and molecular assays are helpful in making a correct diagnosis in morphologically challenging cases. In this review, we present our approach for the diagnosis of breast spindle cell lesions, highlighting the main features of each entity and the potential pitfalls, particularly on CNB. Breast spindle cell lesions are generally classified into two main categories: bland-appearing and malignant-appearing lesions. Each category includes a distinct list of differential diagnoses and a panel of immunohistochemical markers. In bland-appearing lesions, it is important to distinguish fibromatosis-like spindle cell metaplastic breast carcinoma from other benign entities and to distinguish fibromatosis from scar tissue. The malignant-appearing category includes spindle cell metaplastic carcinoma, stroma rich malignant phyllodes tumour, other primary and metastatic malignant spindle cell tumours of the breast, including angiosarcoma and melanoma, and benign mimics such as florid granulation tissue and nodular fasciitis.
Keywords: Approach; Breast; Diagnosis; Immunohistochemistry; Spindle cell lesions.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- van Deurzen CH, Lee AH, Gill MS, Menke-Pluijmers MB, Jager A, Ellis IO, Rakha EA. Metaplastic breast carcinoma: tumour histogenesis or dedifferentiation? J Pathol. 2011;224:434–437. - PubMed
-
- Wang X, Mori I, Tang W, Yang Q, Nakamura M, Nakamura Y, Sato M, et al. Metaplastic carcinoma of the breast: p53 analysis identified the same point mutation in the three histologic components. Mod Pathol. 2001;14:1183–1186. - PubMed
-
- Kaufman MW, Marti JR, Gallager HS, Hoehn JL. Carcinoma of the breast with pseudosarcomatous metaplasia. Cancer. 1984;53:1908–1917. - PubMed
-
- Lim E, Vaillant F, Wu D, Forrest NC, Pal B, Hart AH, Asselin-Labat ML, et al. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat Med. 2009;15:907–913. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

