COVID-19 in lung transplant recipients: A single-center experience
- PMID: 34323353
- PMCID: PMC8420517
- DOI: 10.1111/tid.13700
COVID-19 in lung transplant recipients: A single-center experience
Abstract
Background: Coronavirus disease 2019 (COVID-19) is a global health problem. However, the course of this disease in immunosuppressed patients remains unknown. This study aimed to describe the course of COVID-19 infection and its effects on lung transplant recipients.
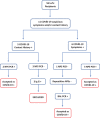
Methods: This was a single-center, retrospective, observational study. The recipients with suspicious symptoms and/or a contact history with infected individuals were diagnosed with COVID-19 by performing a reverse transcription-polymerase chain reaction (RT-PCR) test using samples obtained from the nasopharynx swabs or bronchial lavage. We classified the patients into mild, moderate, and high severity groups according to their clinical conditions. In patients with positive RT-PCR results, cell cycle inhibitor drugs were withdrawn, while steroids were maintained at the same level as in patients without clinical deterioration.
Results: Of the seven recipients diagnosed with COVID-19 infection, one experienced a re-infection. Each recipient had at least one comorbidity. Smell disorder (12.5%), cough/dyspnea (37%), and fever/chills/shivering (37%) were the most frequent symptoms. The mean follow-up time after infection was 108 days. No deaths were recorded due to COVID-19; however, the pulmonary function test values of two recipients were decreased during subsequent follow-ups.
Conclusion: In our small group of transplant recipients with COVID-19, there were two cases of pulmonary function deterioration and a case of re-infection, and no recipient died. It is suggested that steroid therapy should be initiated in the early period in patients with pulmonary opacities.
Keywords: COVID-19; lung transplant; rejection.
© 2021 Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- WHO . https://covid19.who.int/. Accessed March 21, 2021.
-
- Morlacchi LC, Rossetti V, Gigli L, et al. COVID‐19 in lung transplant recipients: a case series from Milan. Italy Transpl Infect Dis. 2020;22:e13356. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical