CD70-specific CAR T cells have potent activity against acute myeloid leukemia without HSC toxicity
- PMID: 34323938
- PMCID: PMC8323977
- DOI: 10.1182/blood.2020008221
CD70-specific CAR T cells have potent activity against acute myeloid leukemia without HSC toxicity
Abstract
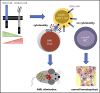
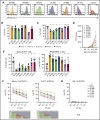
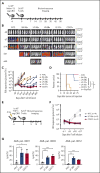
The prognosis of patients with acute myeloid leukemia (AML) remains dismal, highlighting the need for novel innovative treatment strategies. The application of chimeric antigen receptor (CAR) T-cell therapy to patients with AML has been limited, in particular by the lack of a tumor-specific target antigen. CD70 is a promising antigen to target AML, as it is expressed on most leukemic blasts, whereas little or no expression is detectable in normal bone marrow samples. To target CD70 on AML cells, we generated a panel of CD70-CAR T cells that contained a common single-chain variable fragment (scFv) for antigen detection, but differed in size and flexibility of the extracellular spacer and in the transmembrane and the costimulatory domains. These CD70scFv CAR T cells were compared with a CAR construct that contained human CD27, the ligand of CD70 fused to the CD3ζ chain (CD27z). The structural composition of the CAR strongly influenced expression levels, viability, expansion, and cytotoxic capacities of CD70scFv-based CAR T cells, but CD27z-CAR T cells demonstrated superior proliferation and antitumor activity in vitro and in vivo, compared with all CD70scFv-CAR T cells. Although CD70-CAR T cells recognized activated virus-specific T cells (VSTs) that expressed CD70, they did not prevent colony formation by normal hematopoietic stem cells. Thus, CD70-targeted immunotherapy is a promising new treatment strategy for patients with CD70-positive AML that does not affect normal hematopoiesis but will require monitoring of virus-specific T-cell responses.
© 2021 by The American Society of Hematology.
Figures




References
-
- Dinmohamed AG, Visser O, van Norden Y, et al. . Treatment, trial participation and survival in adult acute myeloid leukemia: a population-based study in the Netherlands, 1989-2012. Leukemia. 2016;30(1):24-31. - PubMed
-
- Ishikawa F, Yoshida S, Saito Y, et al. . Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol. 2007;25(11):1315-1321. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

