Waning antibody responses in COVID-19: what can we learn from the analysis of other coronaviruses?
- PMID: 34324165
- PMCID: PMC8319587
- DOI: 10.1007/s15010-021-01664-z
Waning antibody responses in COVID-19: what can we learn from the analysis of other coronaviruses?
Abstract
Objectives: The coronavirus disease 2019 (COVID-19), caused by the novel betacoronavirus severe acute respiratory syndrome 2 (SARS-CoV-2), was declared a pandemic in March 2020. Due to the continuing surge in incidence and mortality globally, determining whether protective, long-term immunity develops after initial infection or vaccination has become critical.
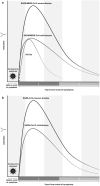
Methods/results: In this narrative review, we evaluate the latest understanding of antibody-mediated immunity to SARS-CoV-2 and to other coronaviruses (SARS-CoV, Middle East respiratory syndrome coronavirus and the four endemic human coronaviruses) in order to predict the consequences of antibody waning on long-term immunity against SARS-CoV-2. We summarise their antibody dynamics, including the potential effects of cross-reactivity and antibody waning on vaccination and other public health strategies. At present, based on our comparison with other coronaviruses we estimate that natural antibody-mediated protection for SARS-CoV-2 is likely to last for 1-2 years and therefore, if vaccine-induced antibodies follow a similar course, booster doses may be required. However, other factors such as memory B- and T-cells and new viral strains will also affect the duration of both natural and vaccine-mediated immunity.
Conclusion: Overall, antibody titres required for protection are yet to be established and inaccuracies of serological methods may be affecting this. We expect that with standardisation of serological testing and studies with longer follow-up, the implications of antibody waning will become clearer.
Keywords: Antibodies; COVID-19; HCoV; MERS; SARS.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- World Health Organisation. Immunization, vaccines and biologicals—the immunological basis for immunization series. https://www.who.int/immunization/documents/immunological_basis_series/en/. Accessed 20 Jan 2021.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

