State-Of-The-Art Quantification of Polymer Solution Viscosity for Plastic Waste Recycling
- PMID: 34324273
- PMCID: PMC8519067
- DOI: 10.1002/cssc.202100876
State-Of-The-Art Quantification of Polymer Solution Viscosity for Plastic Waste Recycling
Abstract
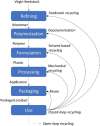
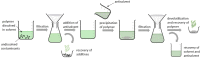
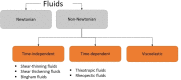
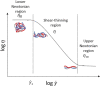
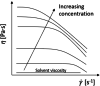
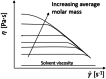
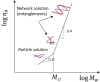
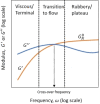
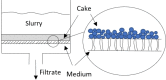
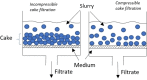
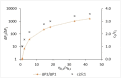
Solvent-based recycling is a promising approach for closed-loop recovery of plastic-containing waste. It avoids the energy cost to depolymerize the plastic but still allows to clean the polymer of contaminants and additives. However, viscosity plays an important role in handling the polymer solutions at high concentrations and in the cleaning steps. This Review addresses the viscosity behavior of polymer solutions, available data, and (mostly algebraic) models developed. The non-Newtonian viscosity models, such as the Carreau and Yasuda-Cohen-Armstrong models, pragmatically describe the viscosity of polymer solutions at different concentrations and shear rate ranges. This Review also describes how viscosity influences filtration and centrifugation processes, which are crucial steps in the cleaning of the polymer and includes a polystyrene/styrene case study.
Keywords: dyes/pigments; plastics recycling; separations; solvent effects; viscosity.
© 2021 The Authors. ChemSusChem published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- PlasticsEurope, Plastics: the Facts 2019, 2019, https://www.plasticseurope.org/en/resources/market-data.
-
- Duan L., D'hooge D. R., Cardon L., Prog. Mater. Sci. 2020, 114, 100617.
-
- Luijsterburg B. J., Jobse P. S., Spoelstra A. B., Goossens J. G. P., Waste Manage. 2016, 54, 53–61. - PubMed
-
- Ragaert K., Delva L., Van Geem K., Waste Manage. 2017, 69, 24–58. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

