High Energy Density Single-Crystal NMC/Li6PS5Cl Cathodes for All-Solid-State Lithium-Metal Batteries
- PMID: 34324288
- PMCID: PMC8397257
- DOI: 10.1021/acsami.1c07952
High Energy Density Single-Crystal NMC/Li6PS5Cl Cathodes for All-Solid-State Lithium-Metal Batteries
Abstract
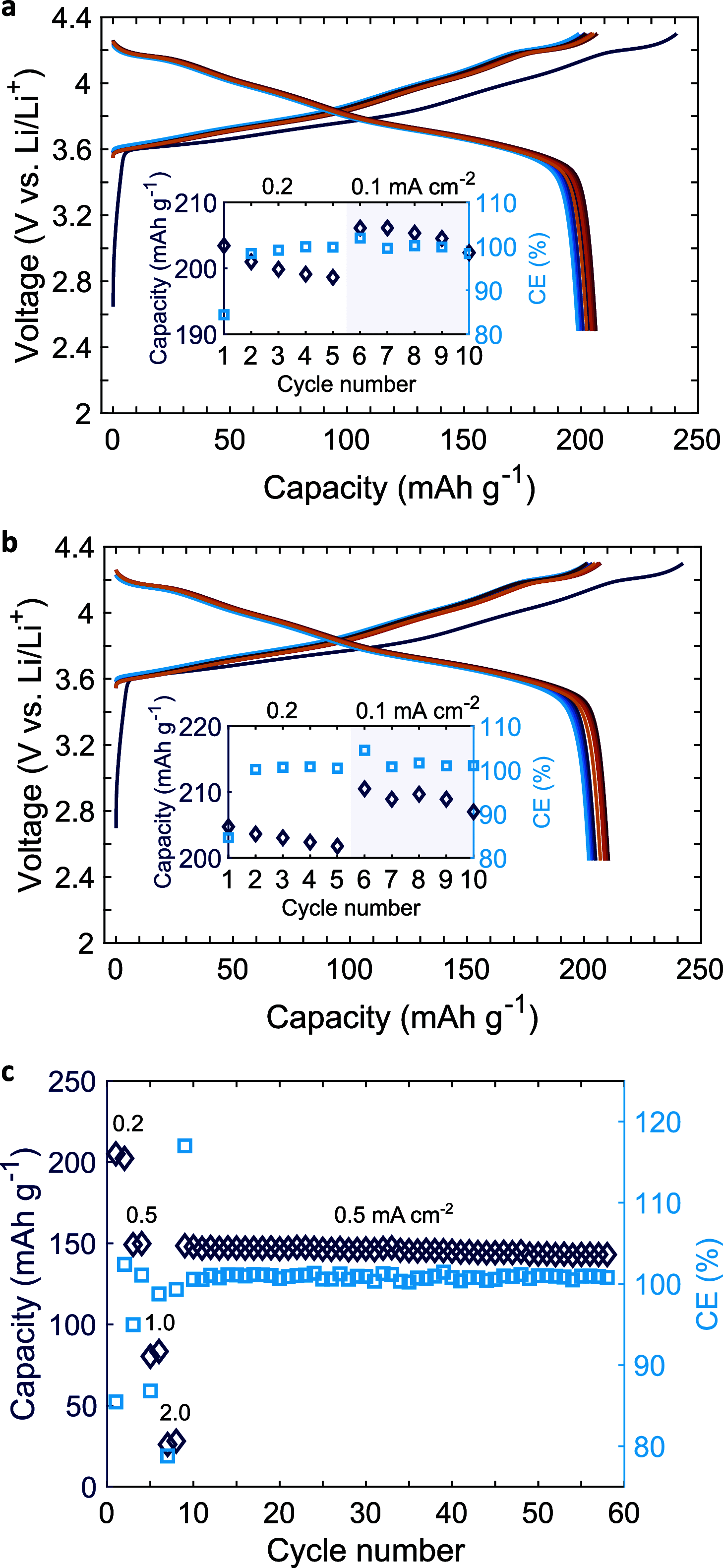
To match the high capacity of metallic anodes, all-solid-state batteries require high energy density, long-lasting composite cathodes such as Ni-Mn-Co (NMC)-based lithium oxides mixed with a solid-state electrolyte (SSE). However in practice, cathode capacity typically fades due to NMC cracking and increasing NMC/SSE interface debonding because of NMC pulverization, which is only partially mitigated by the application of a high cell pressure during cycling. Using smart processing protocols, we report a single-crystal particulate LiNi0.83Mn0.06Co0.11O2 and Li6PS5Cl SSE composite cathode with outstanding discharge capacity of 210 mA h g-1 at 30 °C. A first cycle coulombic efficiency of >85, and >99% thereafter, was achieved despite a 5.5% volume change during cycling. A near-practical discharge capacity at a high areal capacity of 8.7 mA h cm-2 was obtained using an asymmetric anode/cathode cycling pressure of only 2.5 MPa/0.2 MPa.
Keywords: composite cathode; interfacial contact; pressure dependence; single-crystal NMC; solid-state battery; stack pressure; sulfide electrolyte; volume expansion.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Janek J.; Zeier W. G. A Solid Future for Battery Development. Nat. Energy 2016, 1, 16141.10.1038/nenergy.2016.141. - DOI
-
- Pasta M.; Armstrong D.; Brown Z. L.; Bu J.; Castell M. R.; Chen P.; Clocks A.; Corr S. A.; Cussen E. J.; Darnbrough E.; et al. 2020 Roadmap on Solid-State Batteries. J. Phys.: Energy 2020, 2, 032008.10.1088/2515-7655/ab95f4. - DOI
-
- Cao D.; Zhao Y.; Sun X.; Natan A.; Wang Y.; Xiang P.; Wang W.; Zhu H. Processing Strategies to Improve Cell-Level Energy Density of Metal Sulfide Electrolyte-Based All-Solid-State Li Metal Batteries and Beyond. ACS Energy Lett. 2020, 5, 3468–3489. 10.1021/acsenergylett.0c01905. - DOI
-
- Schnell J.; Günther T.; Knoche T.; Vieider C.; Köhler L.; Just A.; Keller M.; Passerini S.; Reinhart G. All-Solid-State Lithium-Ion and Lithium Metal Batteries – Paving the Way to Large-Scale Production. J. Power Sources 2018, 382, 160–175. 10.1016/j.jpowsour.2018.02.062. - DOI
-
- Wenzel S.; Sedlmaier S. J.; Dietrich C.; Zeier W. G.; Janek J. Interfacial Reactivity and Interphase Growth of Argyrodite Solid Electrolytes at Lithium Metal Electrodes. Solid State Ionics 2018, 318, 102–112. 10.1016/j.ssi.2017.07.005. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

