Creatine transporter deficiency impairs stress adaptation and brain energetics homeostasis
- PMID: 34324436
- PMCID: PMC8492331
- DOI: 10.1172/jci.insight.140173
Creatine transporter deficiency impairs stress adaptation and brain energetics homeostasis
Abstract
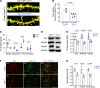
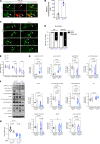
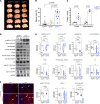
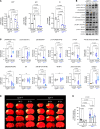
The creatine transporter (CrT) maintains brain creatine (Cr) levels, but the effects of its deficiency on energetics adaptation under stress remain unclear. There are also no effective treatments for CrT deficiency, the second most common cause of X-linked intellectual disabilities. Herein, we examined the consequences of CrT deficiency in brain energetics and stress-adaptation responses plus the effects of intranasal Cr supplementation. We found that CrT-deficient (CrT-/y) mice harbored dendritic spine and synaptic dysgenesis. Nurtured newborn CrT-/y mice maintained baseline brain ATP levels, with a trend toward signaling imbalance between the p-AMPK/autophagy and mTOR pathways. Starvation elevated the signaling imbalance and reduced brain ATP levels in P3 CrT-/y mice. Similarly, CrT-/y neurons and P10 CrT-/y mice showed an imbalance between autophagy and mTOR signaling pathways and greater susceptibility to cerebral hypoxia-ischemia and ischemic insults. Notably, intranasal administration of Cr after cerebral ischemia increased the brain Cr/N-acetylaspartate ratio, partially averted the signaling imbalance, and reduced infarct size more potently than intraperitoneal Cr injection. These findings suggest important functions for CrT and Cr in preserving the homeostasis of brain energetics in stress conditions. Moreover, intranasal Cr supplementation may be an effective treatment for congenital CrT deficiency and acute brain injury.
Keywords: Autophagy; Bioenergetics; Metabolism; Neurological disorders; Neuroscience.
Conflict of interest statement
Figures





References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

