Targeting human langerin promotes HIV-1 specific humoral immune responses
- PMID: 34324611
- PMCID: PMC8354475
- DOI: 10.1371/journal.ppat.1009749
Targeting human langerin promotes HIV-1 specific humoral immune responses
Abstract
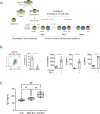
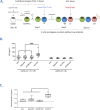
The main avenue for the development of an HIV-1 vaccine remains the induction of protective antibodies. A rationale approach is to target antigen to specific receptors on dendritic cells (DC) via fused monoclonal antibodies (mAb). In mouse and non-human primate models, targeting of skin Langerhans cells (LC) with anti-Langerin mAbs fused with HIV-1 Gag antigen drives antigen-specific humoral responses. The development of these immunization strategies in humans requires a better understanding of early immune events driven by human LC. We therefore produced anti-Langerin mAbs fused with the HIV-1 gp140z Envelope (αLC.Env). First, we show that primary skin human LC and in vitro differentiated LC induce differentiation and expansion of naïve CD4+ T cells into T follicular helper (Tfh) cells. Second, when human LC are pre-treated with αLC.Env, differentiated Tfh cells significantly promote the production of specific IgG by B cells. Strikingly, HIV-Env-specific Ig are secreted by HIV-specific memory B cells. Consistently, we found that receptors and cytokines involved in Tfh differentiation and B cell functions are upregulated by LC during their maturation and after targeting Langerin. Finally, we show that subcutaneous immunization of mice by αLC.Env induces germinal center (GC) reaction in draining lymph nodes with higher numbers of Tfh cells, Env-specific B cells, as well as specific IgG serum levels compared to mice immunized with the non-targeting Env antigen. Altogether, we provide evidence that human LC properly targeted may be licensed to efficiently induce Tfh cell and B cell responses in GC.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: GZ, SZ, and YL are named inventors on patent applications held by Inserm and BIIR concerning Langerin targeting. All other authors have declared that no competing interest exist.
Figures



References
-
- Godot V, Tcherakian C, Gil L, Cervera-Marzal I, Li G, Cheng L, et al.. TLR-9 agonist and CD40-targeting vaccination induces HIV-1 envelope-specific B cells with a diversified immunoglobulin repertoire in humanized mice. PLoS Pathog. 2020;16:1–23. doi: 10.1371/journal.ppat.1009025 - DOI - PMC - PubMed
-
- Cheong C, Choi J, Vitale L, He L-Z, Trumpfheller C, Bozzacco L, et al.. Improved cellular and humoral immune responses in vivo following targeting of HIV Gag to dendritic cells within human anti–human DEC205 monoclonal antibody. Blood. 2010;116:3828–3838. doi: 10.1182/blood-2010-06-288068 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

