Short- and longer-term cancer risks with biologic and targeted synthetic disease-modifying antirheumatic drugs as used against rheumatoid arthritis in clinical practice
- PMID: 34324640
- PMCID: PMC9071561
- DOI: 10.1093/rheumatology/keab570
Short- and longer-term cancer risks with biologic and targeted synthetic disease-modifying antirheumatic drugs as used against rheumatoid arthritis in clinical practice
Abstract
Objective: To estimate the occurrence and relative risks of first-ever-incident non-cutaneous cancer overall and for 16 sites in patients with RA treated with biologic and targeted synthetic DMARDs (b/tsDMARDs), by time since treatment start, attained age, and duration of active treatment.
Methods: This is an observational nationwide and population-based cohort study of patients with RA (n = 69 308), treated with TNF inhibitors (TNFi; adalimumab, certolizumab, etanercept, golimumab, infliximab) or other b/tsDMARDs (abatacept, rituximab, baricitinib, tofacitinib and tocilizumab) compared with RA patients not treated with b/tsDMARDs, and matched general population referents (n = 109 532), 2001-2018. The study was based on prospectively collected data from the Swedish Rheumatology Quality Register and from other registers, linked to the national Swedish Cancer Register. Incidence rates and hazard ratios were estimated via Cox regression adjusted for co-morbidities and other health characteristics.
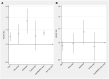
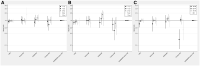
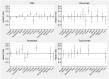
Results: Based on 8633 incident cancers among RA patients, the overall relative risk of cancer with TNFi [hazard ratio (HR) = 1.0] was neither increased nor did it change with time since treatment start, duration of active treatment, or attained age, when compared with b/tsDMARD-naïve RA. For other b/tsDMARDs, we noted no consistent signal of increased overall risks (HRs ranged from 1.0 to 1.2), but there were statistically significant estimates above 1 for abatacept with 2-5 years of active treatment, for older age groups, and between several of the bDMARDs and urinary tract cancer.
Conclusion: TNFis, as used long term in clinical practice against RA, are not linked to increased risks for cancer overall. For other b/tsDMARDs, and for site-specific risks, our results are generally reassuring but contain signals that call for replication.
Keywords: Sweden; b/tsDMARD; cancer; cohort; register; rheumatoid arthritis.
© The Author(s) 2021. Published by Oxford University Press on behalf of the British Society for Rheumatology.
Figures
Comment in
-
Long-term treatment in rheumatoid arthritis: do biological and targeted-synthetic DMARDs increase the risk of malignancy?Rheumatology (Oxford). 2022 May 5;61(5):1758-1759. doi: 10.1093/rheumatology/keab750. Rheumatology (Oxford). 2022. PMID: 34648000 No abstract available.
References
-
- de Visser KE, Eichten A, Coussens LM.. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer 2006;6:24–37. - PubMed
-
- Lebrec H, Ponce R, Preston BD. et al. Tumor necrosis factor, tumor necrosis factor inhibition, and cancer risk. Curr Med Res Opin 2015;31:557–74. - PubMed
-
- Bongartz T, Sutton AJ, Sweeting MJ. et al. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA 2006;295:2275–85. - PubMed
-
- Lopez-Olivo MA, Tayar JH, Martinez-Lopez JA. et al. Risk of malignancies in patients with rheumatoid arthritis treated with biologic therapy: a meta-analysis. Jama 2012;308:898–908. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

