The microbiota coordinates diurnal rhythms in innate immunity with the circadian clock
- PMID: 34324837
- PMCID: PMC8967342
- DOI: 10.1016/j.cell.2021.07.001
The microbiota coordinates diurnal rhythms in innate immunity with the circadian clock
Abstract
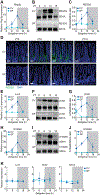
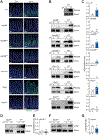
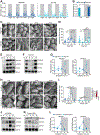
Environmental light cycles entrain circadian feeding behaviors in animals that produce rhythms in exposure to foodborne bacteria. Here, we show that the intestinal microbiota generates diurnal rhythms in innate immunity that synchronize with feeding rhythms to anticipate microbial exposure. Rhythmic expression of antimicrobial proteins was driven by daily rhythms in epithelial attachment by segmented filamentous bacteria (SFB), members of the mouse intestinal microbiota. Rhythmic SFB attachment was driven by the circadian clock through control of feeding rhythms. Mechanistically, rhythmic SFB attachment activated an immunological circuit involving group 3 innate lymphoid cells. This circuit triggered oscillations in epithelial STAT3 expression and activation that produced rhythmic antimicrobial protein expression and caused resistance to Salmonella Typhimurium infection to vary across the day-night cycle. Thus, host feeding rhythms synchronize with the microbiota to promote rhythms in intestinal innate immunity that anticipate exogenous microbial exposure.
Keywords: Antimicrobial proteins; circadian clock; feeding rhythms; foodborne pathogen; innate immunity; innate lymphoid cells; intestine; microbiota.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




Comment in
-
Rhythmic resistance.Nat Rev Microbiol. 2021 Oct;19(10):618. doi: 10.1038/s41579-021-00618-4. Nat Rev Microbiol. 2021. PMID: 34345041 No abstract available.
-
Keeping the clock running: The microbiome as a pivotal timekeeper.Cell Metab. 2021 Aug 3;33(8):1514-1515. doi: 10.1016/j.cmet.2021.07.014. Cell Metab. 2021. PMID: 34348097
References
-
- Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, and Akira S (1998). Targeted disruption of the MyD88 gene results in loss of IL-1-and IL-18-mediated function. Immunity 9, 143–150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

