Translation and validation of the Chinese version of the MD Anderson symptom inventory for measuring perioperative symptom burden in patients with gynecologic cancer
- PMID: 34325677
- PMCID: PMC8320042
- DOI: 10.1186/s12905-021-01415-0
Translation and validation of the Chinese version of the MD Anderson symptom inventory for measuring perioperative symptom burden in patients with gynecologic cancer
Abstract
Background: Gynecologic cancers are among the most prevalent malignancies in China. Cervical and uterine cancer respectively account for the sixth and eighth highest incidence of cancer among Chinese women. Abdominal surgery is one of the important treatment methods for gynecological tumors. However, the tumor- and surgery-related symptom burden are not well studied owing to a lack of a standardized and validated assessment tool in the Chinese population. The study aimed to translate and validate the MD Anderson Symptom Inventory for measuring perioperative symptom burden in gynecologic cancer patients (MDASI-PeriOp-GYN) and examine the utility of the Chinese version of MDASI-PeriOp-GYN.
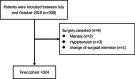
Methods: The MDASI-PeriOp-GYN was translated in a stepwise manner. First, two native speakers independently translated the 9 PeriOp-GYN symptom items. Then the nine items were translated back into English by two different bilingual translators. After discussion and revision, the four translators reached an agreement. Finally, the finalized Chinese version was administered to women with three common gynecologic cancer types (cervical, ovarian, and endometrial cancers) recruited from the gynecological oncology department of Sichuan Cancer Hospital & Institute between July and October 2019. The reliability and validity of the translated version were assessed.
Results: Overall, 324 women with gynecologic cancers were enrolled. Cronbach's α values were 0.826 and 0.735 for the symptom severity and interference scales, respectively. Test-retest reliability values were 0.885, 0.873, and 0.914 for symptom severity, PeriOp-GYN, and interference scales. Significant correlations were found between the MDASI-PeriOp-GYN-C and EORTC QLQ-C30 along with the QLQ-OV28 module (- 0.608-0.871, P < 0.001). Known-group validity was supported by significant differences in the scores of the four scales grouped by time intervals, surgery type, and functional status (all P < 0.01).
Conclusions: The MDASI-PeriOp-GYN-C is a valid and reliable tool for measuring symptoms in Chinese patients undergoing surgery for gynecologic cancers. The tool could be used in clinical practice and clinical trials to instantly gather patients' health and quality of life data.
Keywords: Chinese; Gynecology; Neoplasms; Symptom assessment.
© 2021. The Author(s).
Conflict of interest statement
None of the authors have any conflicts of interest to declare.
Figures
Similar articles
-
Validation and application of a module of the MD Anderson Symptom Inventory for measuring perioperative symptom burden in patients with gynecologic cancer (the MDASI-PeriOp-GYN).Gynecol Oncol. 2019 Mar;152(3):492-500. doi: 10.1016/j.ygyno.2018.11.004. Gynecol Oncol. 2019. PMID: 30876494 Free PMC article.
-
Development of a patient-reported outcome tool for assessing symptom burden during perioperative care in liver surgery: The MDASI-PeriOp-Hep.Eur J Oncol Nurs. 2021 Jun;52:101959. doi: 10.1016/j.ejon.2021.101959. Epub 2021 Apr 28. Eur J Oncol Nurs. 2021. PMID: 33964632 Free PMC article.
-
Translation and validation of M.D. Anderson Symptom Inventory-Thyroid Cancer module in Chinese thyroid cancer patients: a cross-sectional and methodological study.BMC Cancer. 2022 Aug 26;22(1):924. doi: 10.1186/s12885-022-09995-2. BMC Cancer. 2022. PMID: 36028793 Free PMC article.
-
Psychometric validation of the Moroccan version of the EORTC QLQ-C30 in colorectal Cancer patients: cross-sectional study and systematic literature review.BMC Cancer. 2021 Jan 27;21(1):99. doi: 10.1186/s12885-021-07793-w. BMC Cancer. 2021. PMID: 33499819 Free PMC article.
-
Modification of existing patient-reported outcome measures: qualitative development of the MD Anderson Symptom Inventory for malignant pleural mesothelioma (MDASI-MPM).Qual Life Res. 2018 Dec;27(12):3229-3241. doi: 10.1007/s11136-018-1982-5. Epub 2018 Sep 5. Qual Life Res. 2018. PMID: 30187393 Review.
Cited by
-
Symptom burden, psychological distress, and symptom management status in hospitalized patients with advanced cancer: a multicenter study in China.ESMO Open. 2022 Dec;7(6):100595. doi: 10.1016/j.esmoop.2022.100595. Epub 2022 Oct 14. ESMO Open. 2022. PMID: 36252435 Free PMC article.
-
Effects of a Phone-Based Support Program for Women With Breast Cancer Undergoing Chemotherapy: A Pilot Study.SAGE Open Nurs. 2024 Feb 26;10:23779608241231176. doi: 10.1177/23779608241231176. eCollection 2024 Jan-Dec. SAGE Open Nurs. 2024. PMID: 38415216 Free PMC article.
-
Effect of Psychological Care Combined with Traditional Chinese Medicine on Postoperative Psychological Stress Response in Patients with Advanced Cervical Cancer.Evid Based Complement Alternat Med. 2021 Sep 28;2021:5612925. doi: 10.1155/2021/5612925. eCollection 2021. Evid Based Complement Alternat Med. 2021. PMID: 34621324 Free PMC article.
-
Translation and validation of chinese version of MDASI immunotherapy for early-phase trials module: a cross-sectional study.BMC Nurs. 2023 May 22;22(1):176. doi: 10.1186/s12912-023-01217-9. BMC Nurs. 2023. PMID: 37217922 Free PMC article.
References
-
- Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000 ;89(7):1634–1646. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources