Frequent LPA KIV-2 Variants Lower Lipoprotein(a) Concentrations and Protect Against Coronary Artery Disease
- PMID: 34325833
- PMCID: PMC7613585
- DOI: 10.1016/j.jacc.2021.05.037
Frequent LPA KIV-2 Variants Lower Lipoprotein(a) Concentrations and Protect Against Coronary Artery Disease
Abstract
Background: Lipoprotein(a) (Lp(a)) concentrations are a major independent risk factor for coronary artery disease (CAD) and are mainly determined by variation in LPA. Up to 70% of the LPA coding sequence is located in the hypervariable kringle IV type 2 (KIV-2) region. It is hardly accessible by conventional technologies, but may contain functional variants.
Objectives: This study sought to investigate the new, very frequent splicing variant KIV-2 4733G>A on Lp(a) and CAD.
Methods: We genotyped 4733G>A in the GCKD (German Chronic Kidney Disease) study (n = 4,673) by allele-specific polymerase chain reaction, performed minigene assays, identified proxy single nucleotide polymorphisms and used them to characterize its effect on CAD by survival analysis in UK Biobank (n = 440,234). Frequencies in ethnic groups were assessed in the 1000 Genomes Project.
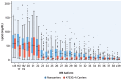
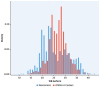
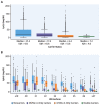
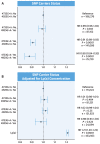
Results: The 4733G>A variant (38.2% carrier frequency) was found in most isoform sizes. It reduces allelic expression without abolishing protein production, lowers Lp(a) by 13.6 mg/dL (95% CI: 12.5-14.7; P < 0.0001) and is the strongest variance-explaining factor after the smaller isoform. Splicing of minigenes was modified. Compound heterozygosity (4.6% of the population) for 4733G>A and 4925G>A, another KIV-2 splicing mutation, reduces Lp(a) by 31.8 mg/dL and most importantly narrows the interquartile range by 9-fold (from 42.1 to 4.6 mg/dL) when compared to the wild type. In UK Biobank 4733G>A alone and compound heterozygosity with 4925G>A reduced HR for CAD by 9% (95% CI: 7%-11%) and 12% (95% CI: 7%-16%) (both P < 0.001). Frequencies in ethnicities differ notably.
Conclusions: Functional variants in the previously inaccessible LPA KIV-2 region cooperate in determining Lp(a) variance and CAD risk. Even a moderate but lifelong genetic Lp(a) reduction translates to a noticeable CAD risk reduction.
Keywords: Mendelian randomization; cardiovascular disease; cohort study; copy number variation; genetic variability; lipoprotein(a).
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding Support and Author Disclosures The study was supported by the Austrian Science Fund (FWF) project P31458-B34 and the D•A•CH Advancement Award Lipidology 2015 (supported by the Christine Katharine Schmitz Foundation) of the D•A•CH-Society for Prevention of Cardiovascular Diseases (to S.C.). The GCKD study is supported by the German Ministry of Education and Research (Bundesministerium für Bildung und Forschung, grants 01ER 0804, 01ER 0818, 01ER 0819, 01ER 0820, and 01ER 0821) and the KfH Foundation for Preventive Medicine (Kuratorium für Heimdialyse und Nierentransplantation e.V.–Stiftung Präventivmedizin) and corporate sponsors (see the GCKD website). Ms Schachtl-Riess has received support from the Dr Legerlotz Foundation. Dr Köttgen has received support from the German Research Foundation (grant KO 3598/5-1). Dr Kronenberg has received support from the Austrian Science Fund (project W-1253DK HOROS); has received lecture fees from Novartis, Amgen, and Kaneka; and has served on the advisory boards of Amgen and Kaneka. Drs Kronenberg and Coassin have received support from the Lipoprotein(a) Center And Research InstitutE [Lp(a)CARE] for their lipoprotein(a) research. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures


Comment in
-
Genetics to the Rescue: Sophisticated Approaches Provide Critical Insights Into the Determination of Lp(a) Levels.J Am Coll Cardiol. 2021 Aug 3;78(5):450-452. doi: 10.1016/j.jacc.2021.06.004. J Am Coll Cardiol. 2021. PMID: 34325834 No abstract available.
References
-
- Tsimikas S. Atest in context: lipoprotein(a):diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol. 2017;69(6):692–711. - PubMed
-
- Langsted A, Kamstrup PR, Nordestgaard BG. High lipoprotein(a) and high risk of mortality. Eur Heart J. 2019;40(33):2760–70. - PubMed
-
- Boffa MB, Koschinsky ML. Oxidized phospholipids as a unifying theory for lipoprotein(a) and cardiovascular disease. Nat Rev Cardiol. 2019;16(5):305–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

