Treatment of tungiasis using a tea tree oil-based gel formulation: protocol for a randomised controlled proof-of-principle trial
- PMID: 34326048
- PMCID: PMC8323357
- DOI: 10.1136/bmjopen-2020-047380
Treatment of tungiasis using a tea tree oil-based gel formulation: protocol for a randomised controlled proof-of-principle trial
Abstract
Introduction: Tungiasis (sand flea disease or jigger infestation) is a neglected tropical disease caused by penetration of female sand fleas, Tunga penetrans, in the skin. The disease inflicts immense pain and suffering on millions of people, particularly children, in Latin America, the Caribbean and sub-Saharan Africa. Currently, there is no standard treatment for tungiasis, and a simple, safe and effective tungiasis treatment option is required. Tea tree oil (TTO) has long been used as a parasiticidal agent against ectoparasites such as headlice, mites and fleas with proven safety and efficacy data. However, current data are insufficient to warrant a recommendation for its use in tungiasis. This trial aims to generate these data by comparing the safety and efficacy of a 5% (v/w) TTO proprietary gel formulation with 0.05% (w/v) potassium permanganate (KMnO4) solution for tungiasis treatment.
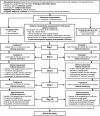
Methods and analysis: This trial is a randomised controlled trial (RCT) in primary schools (n=8) in South-Western Kenya. The study will include school children (n=88) aged 6-15 years with a confirmed diagnosis of tungiasis. The participants will be randomised in a 1:1 ratio to receive a 3-day two times a day treatment of either 5% TTO gel or 0.05% KMnO4 solution. Two viable embedded sandflea lesions per participant will be targeted and the viability of these lesions will be followed throughout the study using a digital handheld microscope. The primary outcome is the proportion of observed viable embedded sand fleas that have lost viability (non-viable lesions) by day 10 (9 days after first treatment). Secondary outcomes include improvement in acute tungiasis morbidities assessed using a validated severity score for tungiasis, safety assessed through adverse events and product acceptability assessed by interviewing the participants to rate the treatment in terms of effectiveness, side effects, convenience, suitability and overall satisfaction.
Ethics and dissemination: The trial protocol has been reviewed and approved by the University of Canberra Human Research Ethics Committee (HREC-2019-2114). The findings of the study will be presented at scientific conferences and published in a peer-reviewed journal.
Trial registration numbers: Australian New Zealand Clinical Trials Registry (ACTRN12619001610123); PACTR202003651095100 and U1111-1243-2294.
Keywords: complementary medicine; dermatology; infectious diseases; infectious diseases & infestations; paediatric dermatology.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- WHO/Department of control of neglected tropical diseases . Recognizing neglected tropical diseases through changes on the skin: A training guide for front-line health workers [internet]. Geneva, Swizerland: WHO; 2018 [cited 2018 June 1]. Available: http://www.who.int/neglected_diseases/resources/9789241513531/en/ [Accessed 9 Oct 2018].
-
- Muehlen M, Heukelbach J, Wilcke T, et al. Investigations on the biology, epidemiology, pathology and control of Tunga penetrans in Brazil. II. prevalence, parasite load and topographic distribution of lesions in the population of a traditional fishing village. Parasitol Res 2003;90:449–55. 10.1007/s00436-003-0877-7 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources