Intercellular Arc Signaling Regulates Vasodilation
- PMID: 34326146
- PMCID: PMC8445061
- DOI: 10.1523/JNEUROSCI.0440-21.2021
Intercellular Arc Signaling Regulates Vasodilation
Abstract
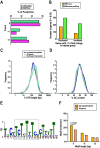
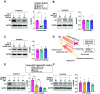
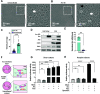
Injury responses require communication between different cell types in the skin. Sensory neurons contribute to inflammation and can secrete signaling molecules that affect non-neuronal cells. Despite the pervasive role of translational regulation in nociception, the contribution of activity-dependent protein synthesis to inflammation is not well understood. To address this problem, we examined the landscape of nascent translation in murine dorsal root ganglion (DRG) neurons treated with inflammatory mediators using ribosome profiling. We identified the activity-dependent gene, Arc, as a target of translation in vitro and in vivo Inflammatory cues promote local translation of Arc in the skin. Arc-deficient male mice display exaggerated paw temperatures and vasodilation in response to an inflammatory challenge. Since Arc has recently been shown to be released from neurons in extracellular vesicles (EVs), we hypothesized that intercellular Arc signaling regulates the inflammatory response in skin. We found that the excessive thermal responses and vasodilation observed in Arc defective mice are rescued by injection of Arc-containing EVs into the skin. Our findings suggest that activity-dependent production of Arc in afferent fibers regulates neurogenic inflammation potentially through intercellular signaling.SIGNIFICANCE STATEMENT Nociceptors play prominent roles in pain and inflammation. We examined rapid changes in the landscape of nascent translation in cultured dorsal root ganglia (DRGs) treated with a combination of inflammatory mediators using ribosome profiling. We identified several hundred transcripts subject to rapid preferential translation. Among them is the immediate early gene (IEG) Arc. We provide evidence that Arc is translated in afferent fibers in the skin. Arc-deficient mice display several signs of exaggerated inflammation which is normalized on injection of Arc containing extracellular vesicles (EVs). Our work suggests that noxious cues can trigger Arc production by nociceptors which in turn constrains neurogenic inflammation in the skin.
Keywords: Arc; DRG; neuroinflamation; nociceptors; translational control.
Copyright © 2021 the authors.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
