Tryptophan potentiates CD8+ T cells against cancer cells by TRIP12 tryptophanylation and surface PD-1 downregulation
- PMID: 34326168
- PMCID: PMC8323461
- DOI: 10.1136/jitc-2021-002840
Tryptophan potentiates CD8+ T cells against cancer cells by TRIP12 tryptophanylation and surface PD-1 downregulation
Abstract
Background: Tryptophan catabolites suppress immunity. Therefore, blocking tryptophan catabolism with indoleamine 2,3-dioxygenase (IDO) inhibitors is pursued as an anticancer strategy.
Methods: The intracellular level of tryptophan and kynurenine was detected by mass spectrum analysis. The effect of tryptophan and IDO inhibitors on cell surface programmed cell death protein 1 (PD-1) level were measured by flow cytometry. A set of biochemical analyses were used to figure out the underlying mechanism. In vitro co-culture system, syngeneic mouse models, immunofluorescent staining, and flow cytometry analysis were employed to investigate the role of tryptophan and IDO inhibitor in regulating the cytotoxicity of CD8+ T cells.
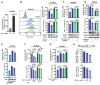
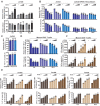
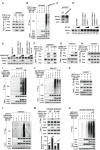
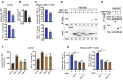
Results: Here, we reported that IDO inhibitors activated CD8+ T cells also by accumulating tryptophan that downregulated PD-1. Tryptophan and IDO inhibitors administration, both increased intracellular tryptophan, and tryptophanyl-tRNA synthetase (WARS) overexpression decreased Jurkat and mice CD8+ T cell surface PD-1. Mechanistically, WARS tryptophanylated lysine 1136 of and activated E3 ligase TRIP12 to degrade NFATc1, a PD-1 transcription activator. SIRT1 de-tryptophanylated TRIP12 and reversed the effects of tryptophan and WARS on PD-1. Tryptophan or IDO inhibitors potentiated CD8+ T cells to induce apoptosis of co-cultured cancer cells, increased cancer-infiltrating CD8+ T cells and slowed down tumor growth of lung cancer in mice.
Conclusions: Our results revealed the immune-activating efficacy of tryptophan, and suggested tryptophan supplemental may benefit IDO inhibitors and PD-1 blockade during anticancer treatments.
Keywords: CD8-positive T-lymphocytes; immunotherapy; indoleamine 2,3-dioxygenase.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: No, there are no competing interests.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
