Pseudoboehmite as a drug delivery system for acyclovir
- PMID: 34326377
- PMCID: PMC8322319
- DOI: 10.1038/s41598-021-94325-y
Pseudoboehmite as a drug delivery system for acyclovir
Abstract
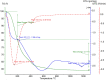
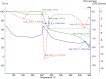
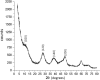
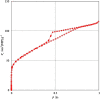
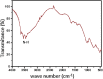
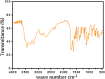
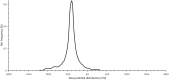
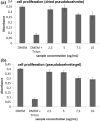
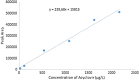
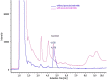
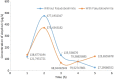
Herpes simplex virus is among the most prevalent sexually transmitted infections. Acyclovir is a potent, selective inhibitor of herpes viruses and it is indicated for the treatment and management of recurrent cold sores on the lips and face, genital herpes, among other diseases. The problem of the oral bioavailability of acyclovir is limited because of the low permeability across the gastrointestinal membrane. The use of nanoparticles of pseudoboehmite as a drug delivery system in vitro assays is a promising approach to further the permeability of acyclovir release. Here we report the synthesis of high purity pseudoboehmite from aluminium nitrate and ammonium hydroxide containing nanoparticles, using the sol-gel method, as a drug delivery system to improve the systemic bioavailability of acyclovir. The presence of pseudoboehmite nanoparticles were verified by infrared spectroscopy, transmission electron microscopy, and X-ray diffraction techniques. In vivo tests were performed with Wistar rats to compare the release of acyclovir, with and without the addition of pseudoboehmite. The administration of acyclovir with the addition of pseudoboehmite increased the drug content by 4.6 times in the plasma of Wistar rats after 4 h administration. We determined that the toxicity of pseudoboehmite is low up to 10 mg/mL, in gel and the dried pseudoboehmite nanoparticles.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2017 (Department of Health and Human Services, 2018). 10.15620/cdc.59237.
-
- Ikawa Y, Fujiki T, Nishimura R, Noguchi K, Koshino E, Fujiki A, Fukuda M, Kuroda R, Mase S, Araki R, Maeba H, Shiraki K, Yachie A. Improvement of refractory acyclovir-resistant herpes simplex virus type 1 infection by continuous acyclovir administration. J. Infect. Chemother. 2019;25(1):65–67. doi: 10.1016/j.jiac.2018.07.004. - DOI - PubMed
-
- Baumrin E, Cheng MP, Kanjilal S, Ho VT, Issa NC, Baden LR. Severe herpes zoster requiring intravenous antiviral treatment in allogeneic hematopoietic cell transplantation recipients on standard acyclovir prophylaxis. Biol. Blood Marrow Transplant. 2019 doi: 10.1016/j.bbmt.2019.04.015. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

