A bioimpedance-based monitor for real-time detection and identification of secondary brain injury
- PMID: 34326387
- PMCID: PMC8322167
- DOI: 10.1038/s41598-021-94600-y
A bioimpedance-based monitor for real-time detection and identification of secondary brain injury
Abstract
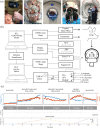
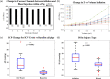
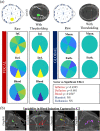
Secondary brain injury impacts patient prognosis and can lead to long-term morbidity and mortality in cases of trauma. Continuous monitoring of secondary injury in acute clinical settings is primarily limited to intracranial pressure (ICP); however, ICP is unable to identify essential underlying etiologies of injury needed to guide treatment (e.g. immediate surgical intervention vs medical management). Here we show that a novel intracranial bioimpedance monitor (BIM) can detect onset of secondary injury, differentiate focal (e.g. hemorrhage) from global (e.g. edema) events, identify underlying etiology and provide localization of an intracranial mass effect. We found in an in vivo porcine model that the BIM detected changes in intracranial volume down to 0.38 mL, differentiated high impedance (e.g. ischemic) from low impedance (e.g. hemorrhagic) injuries (p < 0.001), separated focal from global events (p < 0.001) and provided coarse 'imaging' through localization of the mass effect. This work presents for the first time the full design, development, characterization and successful implementation of an intracranial bioimpedance monitor. This BIM technology could be further translated to clinical pathologies including but not limited to traumatic brain injury, intracerebral hemorrhage, stroke, hydrocephalus and post-surgical monitoring.
© 2021. The Author(s).
Conflict of interest statement
R.J.H. and P.K.M. are co-inventors on US Patent # 8,764,672 B2: System, method and device for monitoring the condition of an internal organ. Scope of patent covered in manuscript: Use of intracranial electrode to overcome high impedance skull. RyTek Medical Inc. is a startup spun out of Dartmouth College and solely owned by R.J.H. Rytek Medical provided partial funding for this work through an NIH STTR Phase 1 grant (#1R41NS100313-01) sub-award made to Dartmouth College. All other authors declare no competing interests.
Figures




References
-
- Carney N, et al. Guidelines for the management of severe traumatic brain injury, Fourth Edition. Neurosurgery. 2016;80:1. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

