Torpor enhances synaptic strength and restores memory performance in a mouse model of Alzheimer's disease
- PMID: 34326412
- PMCID: PMC8322095
- DOI: 10.1038/s41598-021-94992-x
Torpor enhances synaptic strength and restores memory performance in a mouse model of Alzheimer's disease
Abstract
Hibernation induces neurodegeneration-like changes in the brain, which are completely reversed upon arousal. Hibernation-induced plasticity may therefore be of great relevance for the treatment of neurodegenerative diseases, but remains largely unexplored. Here we show that a single torpor and arousal sequence in mice does not induce dendrite retraction and synapse loss as observed in seasonal hibernators. Instead, it increases hippocampal long-term potentiation and contextual fear memory. This is accompanied by increased levels of key postsynaptic proteins and mitochondrial complex I and IV proteins, indicating mitochondrial reactivation and enhanced synaptic plasticity upon arousal. Interestingly, a single torpor and arousal sequence was also sufficient to restore contextual fear memory in an APP/PS1 mouse model of Alzheimer's disease. Our study demonstrates that torpor in mice evokes an exceptional state of hippocampal plasticity and that naturally occurring plasticity mechanisms during torpor provide an opportunity to identify unique druggable targets for the treatment of cognitive impairment.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

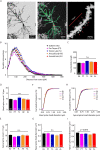
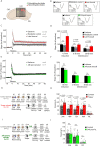


Similar articles
-
Mitochondrial Targeting against Alzheimer's Disease: Lessons from Hibernation.Cells. 2023 Dec 20;13(1):12. doi: 10.3390/cells13010012. Cells. 2023. PMID: 38201215 Free PMC article. Review.
-
Torpor induces reversible tau hyperphosphorylation and accumulation in mice expressing human tau.Acta Neuropathol Commun. 2024 Jun 4;12(1):86. doi: 10.1186/s40478-024-01800-4. Acta Neuropathol Commun. 2024. PMID: 38835043 Free PMC article.
-
The flavonoid baicalein rescues synaptic plasticity and memory deficits in a mouse model of Alzheimer's disease.Behav Brain Res. 2016 Sep 15;311:309-321. doi: 10.1016/j.bbr.2016.05.052. Epub 2016 May 24. Behav Brain Res. 2016. PMID: 27233830
-
Brain inflammatory cytokines and microglia morphology changes throughout hibernation phases in Syrian hamster.Brain Behav Immun. 2018 Feb;68:17-22. doi: 10.1016/j.bbi.2017.10.009. Epub 2017 Oct 14. Brain Behav Immun. 2018. PMID: 29038037
-
[Effects of amyloid β-protein on hippocampal long-term potentiation].Sheng Li Xue Bao. 2010 Dec 25;62(6):479-88. Sheng Li Xue Bao. 2010. PMID: 21170492 Review. Chinese.
Cited by
-
Hippocampal neuroimmune response in mice undergoing serial daily torpor induced by calorie restriction.Front Neuroanat. 2024 Apr 15;18:1334206. doi: 10.3389/fnana.2024.1334206. eCollection 2024. Front Neuroanat. 2024. PMID: 38686173 Free PMC article.
-
The cold truth: torpor as a confound in studies of caloric restriction.J Comp Physiol B. 2025 Jun;195(3):263-276. doi: 10.1007/s00360-025-01616-1. Epub 2025 Jun 9. J Comp Physiol B. 2025. PMID: 40488879 Free PMC article. Review.
-
Mitochondrial Targeting against Alzheimer's Disease: Lessons from Hibernation.Cells. 2023 Dec 20;13(1):12. doi: 10.3390/cells13010012. Cells. 2023. PMID: 38201215 Free PMC article. Review.
-
Torpor induces reversible tau hyperphosphorylation and accumulation in mice expressing human tau.Acta Neuropathol Commun. 2024 Jun 4;12(1):86. doi: 10.1186/s40478-024-01800-4. Acta Neuropathol Commun. 2024. PMID: 38835043 Free PMC article.
-
Opportunities and barriers to translating the hibernation phenotype for neurocritical care.Front Neurol. 2023 Jan 27;14:1009718. doi: 10.3389/fneur.2023.1009718. eCollection 2023. Front Neurol. 2023. PMID: 36779060 Free PMC article. Review.
References
-
- Popov VI, Medvedev NI, Patrushev IV, Ignat’ev DA, Morenkov ED, Stewart MG. Reversible reduction in dendritic spines in CA1 of rat and ground squirrel subjected to hypothermia–normothermia in vivo: A three-dimensional electron microscope study. Neuroscience. 2007;149(3):549–560. doi: 10.1016/j.neuroscience.2007.07.059. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

