Intranasal insulin rescues repeated anesthesia-induced deficits in synaptic plasticity and memory and prevents apoptosis in neonatal mice via mTORC1
- PMID: 34326413
- PMCID: PMC8322102
- DOI: 10.1038/s41598-021-94849-3
Intranasal insulin rescues repeated anesthesia-induced deficits in synaptic plasticity and memory and prevents apoptosis in neonatal mice via mTORC1
Abstract
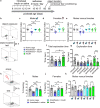
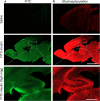
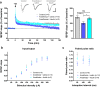
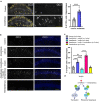
Long-lasting cognitive impairment in juveniles undergoing repeated general anesthesia has been observed in numerous preclinical and clinical studies, yet, the underlying mechanisms remain unknown and no preventive treatment is available. We found that daily intranasal insulin administration to juvenile mice for 7 days prior to repeated isoflurane anesthesia rescues deficits in hippocampus-dependent memory and synaptic plasticity in adulthood. Moreover, intranasal insulin prevented anesthesia-induced apoptosis of hippocampal cells, which is thought to underlie cognitive impairment. Inhibition of the mechanistic target of rapamycin complex 1 (mTORC1), a major intracellular effector of insulin receptor, blocked the beneficial effects of intranasal insulin on anesthesia-induced apoptosis. Consistent with this finding, mice lacking mTORC1 downstream translational repressor 4E-BP2 showed no induction of repeated anesthesia-induced apoptosis. Our study demonstrates that intranasal insulin prevents general anesthesia-induced apoptosis of hippocampal cells, and deficits in synaptic plasticity and memory, and suggests that the rescue effect is mediated via mTORC1/4E-BP2 signaling.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

