Sensitive assay design for detection of anti-drug antibodies to biotherapeutics that lack an immunoglobulin Fc domain
- PMID: 34326436
- PMCID: PMC8322160
- DOI: 10.1038/s41598-021-95055-x
Sensitive assay design for detection of anti-drug antibodies to biotherapeutics that lack an immunoglobulin Fc domain
Abstract
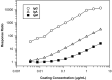
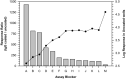
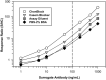
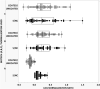
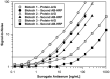
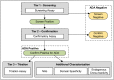
Today the evaluation of unwanted immunogenicity is a key component in the clinical safety evaluation of new biotherapeutic drugs and macromolecular delivery strategies. However, the evolving structural complexity in contemporary biotherapeutics creates a need for on-going innovation in assay designs for reliable detection of anti-drug antibodies, especially for biotherapeutics that may not be well-suited for testing by a bridging assay. We, therefore, initiated systematic optimization of the direct binding assay to adapt it for routine use in regulatory-compliant assays of serum anti-drug antibodies. Accordingly, we first prepared a SULFO-TAG labeled conjugate of recombinant Protein-A/G to create a sensitive electrochemiluminescent secondary detection reagent with broad reactivity to antibodies across many species. Secondly, we evaluated candidate blocker-diluents to identify ones producing the highest signal-to-noise response ratios. Lastly, we introduced use of the ratio of signal responses in biotherapeutic-coated and uncoated wells as a data transformation strategy to identify biological outliers. This alternative data normalization approach improved normality, reduced skewness, and facilitated application of a parametric screening cut point. We believe the optimized direct binding assay design employing SULFO-TAG labeled Protein-A/G represents a useful analytical design for detecting serum ADA to biotherapeutics that lack an immunoglobulin Fc domain.
© 2021. The Author(s).
Conflict of interest statement
All co-authors are employees of B2S Life Sciences (Franklin, IN) and have no conflicts of interest to declare. No writing assistance was used to prepare this manuscript.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

