Mice learn multi-step routes by memorizing subgoal locations
- PMID: 34326540
- PMCID: PMC7617975
- DOI: 10.1038/s41593-021-00884-8
Mice learn multi-step routes by memorizing subgoal locations
Abstract
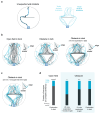
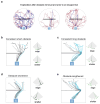
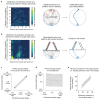
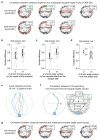
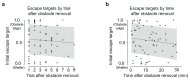
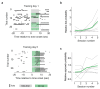
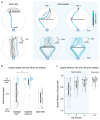
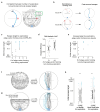
The behavioral strategies that mammals use to learn multi-step routes are unknown. In this study, we investigated how mice navigate to shelter in response to threats when the direct path is blocked. Initially, they fled toward the shelter and negotiated obstacles using sensory cues. Within 20 min, they spontaneously adopted a subgoal strategy, initiating escapes by running directly to the obstacle's edge. Mice continued to escape in this manner even after the obstacle had been removed, indicating use of spatial memory. However, standard models of spatial learning-habitual movement repetition and internal map building-did not explain how subgoal memories formed. Instead, mice used a hybrid approach: memorizing salient locations encountered during spontaneous 'practice runs' to the shelter. This strategy was also used during a geometrically identical food-seeking task. These results suggest that subgoal memorization is a fundamental strategy by which rodents learn efficient multi-step routes in new environments.
© 2021. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
The authors declare no competing interests.
Figures






Comment in
-
A step-by-step guide home.Nat Neurosci. 2021 Sep;24(9):1193-1195. doi: 10.1038/s41593-021-00893-7. Nat Neurosci. 2021. PMID: 34326539 No abstract available.
References
-
- Lima SL, Dill LM. Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool. 1990;68:619–640.
-
- Drickamer Lee C, Stuart J. Peromyscus: Snow Tracking and Possible Cues Used for Navigation. American Midland Naturalist. 1984;111:202.
-
- McMillan BR, Kaufman DW. Travel path characteristics for free-living white-footed mice (Peromyscus leucopus) Can J Zool. 1995;73:1474–1478.
-
- Benhamou S. An analysis of movements of the wood mouse Apodemus sylvaticus in its home range. Behavioural Processes. 1991;22:235–250. - PubMed
-
- Thompson SD. Spatial Utilization and Foraging Behavior of the Desert Woodrat, Neotoma lepida lepida. Journal of Mammalogy. 1982;63:570–581.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

