Pharmacological Modulation of Ion Channels for the Treatment of Cystic Fibrosis
- PMID: 34326672
- PMCID: PMC8316759
- DOI: 10.2147/JEP.S255377
Pharmacological Modulation of Ion Channels for the Treatment of Cystic Fibrosis
Abstract
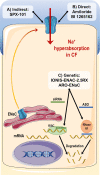
Cystic fibrosis (CF) is a life-shortening monogenic disease caused by mutations in the gene encoding the CF transmembrane conductance regulator (CFTR) protein, an anion channel that transports chloride and bicarbonate across epithelia. Despite clinical progress in delaying disease progression with symptomatic therapies, these individuals still develop various chronic complications in lungs and other organs, which significantly restricts their life expectancy and quality of life. The development of high-throughput assays to screen drug-like compound libraries have enabled the discovery of highly effective CFTR modulator therapies. These novel therapies target the primary defect underlying CF and are now approved for clinical use for individuals with specific CF genotypes. However, the clinically approved modulators only partially reverse CFTR dysfunction and there is still a considerable number of individuals with CF carrying rare CFTR mutations who remain without any effective CFTR modulator therapy. Accordingly, additional efforts have been pursued to identify novel and more potent CFTR modulators that may benefit a larger CF population. The use of ex vivo individual-derived specimens has also become a powerful tool to evaluate novel drugs and predict their effectiveness in a personalized medicine approach. In addition to CFTR modulators, pro-drugs aiming at modulating alternative ion channels/transporters are under development to compensate for the lack of CFTR function. These therapies may restore normal mucociliary clearance through a mutation-agnostic approach (ie, independent of CFTR mutation) and include inhibitors of the epithelial sodium channel (ENaC), modulators of the calcium-activated channel transmembrane 16A (TMEM16, or anoctamin 1) or of the solute carrier family 26A member 9 (SLC26A9), and anionophores. The present review focuses on recent progress and challenges for the development of ion channel/transporter-modulating drugs for the treatment of CF.
Keywords: CFTR modulators; ENaC; SLC26A9; TMEM16A; anionophores; drug development; precision medicine.
© 2021 Pinto et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

