Immune-Related Meningoencephalitis following Nivolumab in Metastatic Renal Cell Carcinoma
- PMID: 34326741
- PMCID: PMC8299396
- DOI: 10.1159/000513001
Immune-Related Meningoencephalitis following Nivolumab in Metastatic Renal Cell Carcinoma
Abstract
While immunotherapy with nivolumab is promising for patients with renal cell carcinoma (RCC), overactivation of the immune system can lead to serious side effects. Immune-related meningoencephalitis without a viral or microbial etiology is a rare complication that may occur in patients treated with checkpoint inhibitors (CPI). Herein, we report a 66-year-old man who underwent a partial nephrectomy which revealed a papillary RCC with clear cell component. Three years later, an abdomen and pelvic CT revealed metastatic lesions in the left psoas muscle and in the left 12th rib. The patient was treated with pazopanib which was discontinued after 2 weeks due to significant hepatic and renal toxicity. He subsequently started sunitinib. Two months later, a chest, abdomen, and pelvic CT demonstrated progressive metastatic RCC in the retroperitoneal mass of the left psoas muscle and paraspinal musculature as well as a left renal mass. The patient was treated with 7 cycles of the CPI nivolumab. He was subsequently hospitalized for 3 weeks after experiencing bilateral lower extremity weakness, lethargy, several falls, hyperthermia, confusion, and gait abnormalities. A CSF analysis demonstrated a lymphocyte pleocytosis with elevated protein and no bacterial or viral growth. The patient was treated with high-dose steroids after which his symptoms resolved. Chest, abdomen, and pelvic CT scans over the next 3 years revealed no evidence of metastatic disease, reflecting a progression-free survival of 40 months. We highlight the unique case of a patient with metastatic RCC who experienced immune-related meningoencephalitis following immunotherapy with nivolumab. Medical oncologists should be alert to the potential development of immune-related encephalitis in patients treated with nivolumab and should promptly diagnose and treat this concerning condition. The excellent oncologic outcome of this case emphasizes the need for continued aggressive measures for management of CNS toxicity resulting from CPI therapy.
Keywords: Checkpoint inhibitor; Immunotherapy; Meningitis; Meningoencephalitis; Nivolumab; Oncology; Renal cell carcinoma.
Copyright © 2021 by S. Karger AG, Basel.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

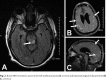
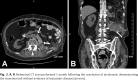
References
-
- American Cancer Society. Key statistics for lung cancer. Accessed October 29, 2020. https://www.cancer.org/cancer/non-small-cell-lung-cancer/about/key-stati....
-
- Baraibar I, Melero I, Ponz-Sarvise M, Castanon E. Safety and tolerability of immune checkpoint inhibitors (PD-1 and PD-L1) in cancer. Drug Saf. 2019;42((2)):281–94. - PubMed
-
- De Giorgi U, Cartenì G, Giannarelli D, Basso U, Galli L, Cortesi E, et al. Safety and efficacy of nivolumab for metastatic renal cell carcinoma: real-world results from an expanded access programme. BJU Int. 2019;123((1)):98–105. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

