New Mammalian Glycerol-3-Phosphate Phosphatase: Role in β-Cell, Liver and Adipocyte Metabolism
- PMID: 34326816
- PMCID: PMC8313997
- DOI: 10.3389/fendo.2021.706607
New Mammalian Glycerol-3-Phosphate Phosphatase: Role in β-Cell, Liver and Adipocyte Metabolism
Abstract
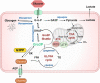
Cardiometabolic diseases, including type 2 diabetes, obesity and non-alcoholic fatty liver disease, have enormous impact on modern societies worldwide. Excess nutritional burden and nutri-stress together with sedentary lifestyles lead to these diseases. Deranged glucose, fat, and energy metabolism is at the center of nutri-stress, and glycolysis-derived glycerol-3-phosphate (Gro3P) is at the crossroads of these metabolic pathways. Cellular levels of Gro3P can be controlled by its synthesis, utilization or hydrolysis. The belief that mammalian cells do not possess an enzyme that hydrolyzes Gro3P, as in lower organisms and plants, is challenged by our recent work showing the presence of a Gro3P phosphatase (G3PP) in mammalian cells. A previously described phosphoglycolate phosphatase (PGP) in mammalian cells, with no established physiological function, has been shown to actually function as G3PP, under physiological conditions, particularly at elevated glucose levels. In the present review, we summarize evidence that supports the view that G3PP plays an important role in the regulation of gluconeogenesis and fat storage in hepatocytes, glucose stimulated insulin secretion and nutri-stress in β-cells, and lipogenesis in adipocytes. We provide a balanced perspective on the pathophysiological significance of G3PP in mammals with specific reference to cardiometabolic diseases.
Keywords: cardiometabolic diseases; glycerol-3-phosphate phosphatase; glycerolipid/free fatty acid cycle; insulin secretion; nutri-stress; obesity; phosphoglycolate phosphatase; type 2 diabetes.
Copyright © 2021 Possik, Al-Mass, Peyot, Ahmad, Al-Mulla, Madiraju and Prentki.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

