The Phage Nucleus and PhuZ Spindle: Defining Features of the Subcellular Organization and Speciation of Nucleus-Forming Jumbo Phages
- PMID: 34326818
- PMCID: PMC8314001
- DOI: 10.3389/fmicb.2021.641317
The Phage Nucleus and PhuZ Spindle: Defining Features of the Subcellular Organization and Speciation of Nucleus-Forming Jumbo Phages
Abstract
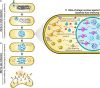
Bacteriophages and their bacterial hosts are ancient organisms that have been co-evolving for billions of years. Some jumbo phages, those with a genome size larger than 200 kilobases, have recently been discovered to establish complex subcellular organization during replication. Here, we review our current understanding of jumbo phages that form a nucleus-like structure, or "Phage Nucleus," during replication. The phage nucleus is made of a proteinaceous shell that surrounds replicating phage DNA and imparts a unique subcellular organization that is temporally and spatially controlled within bacterial host cells by a phage-encoded tubulin (PhuZ)-based spindle. This subcellular architecture serves as a replication factory for jumbo Pseudomonas phages and provides a selective advantage when these replicate in some host strains. Throughout the lytic cycle, the phage nucleus compartmentalizes proteins according to function and protects the phage genome from host defense mechanisms. Early during infection, the PhuZ spindle positions the newly formed phage nucleus at midcell and, later in the infection cycle, the spindle rotates the nucleus while delivering capsids and distributing them uniformly on the nuclear surface, where they dock for DNA packaging. During the co-infection of two different nucleus-forming jumbo phages in a bacterial cell, the phage nucleus establishes Subcellular Genetic Isolation that limits the potential for viral genetic exchange by physically separating co-infection genomes, and the PhuZ spindle causes Virogenesis Incompatibility, whereby interacting components from two diverging phages negatively affect phage reproduction. Thus, the phage nucleus and PhuZ spindle are defining cell biological structures that serve roles in both the life cycle of nucleus-forming jumbo phages and phage speciation.
Keywords: Anti CRISPR mechanism; PhuZ; jumbo phage; nucleus-like compartment; phage nucleus; spindle-like structure; subcellular organization; viral speciation.
Copyright © 2021 Chaikeeratisak, Birkholz and Pogliano.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

