Real-world Effectiveness and Tolerability of Monoclonal Antibody Therapy for Ambulatory Patients With Early COVID-19
- PMID: 34327256
- PMCID: PMC8314951
- DOI: 10.1093/ofid/ofab331
Real-world Effectiveness and Tolerability of Monoclonal Antibody Therapy for Ambulatory Patients With Early COVID-19
Abstract
Background: Neutralizing monoclonal antibodies (MAbs) are a promising therapy for early coronavirus disease 2019 (COVID-19), but their effectiveness has not been confirmed in a real-world setting.
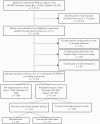
Methods: In this quasi-experimental pre-/postimplementation study, we estimated the effectiveness of MAb treatment within 7 days of symptom onset in high-risk ambulatory adults with COVID-19. The primary outcome was a composite of emergency department visits or hospitalizations within 14 days of positive test. Secondary outcomes included adverse events and 14-day mortality. The average treatment effect in the treated for MAb therapy was estimated using inverse probability of treatment weighting and the impact of MAb implementation using propensity-weighted interrupted time series analysis.
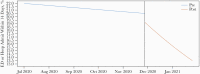
Results: Pre-implementation (July-November 2020), 7404 qualifying patients were identified. Postimplementation (December 2020-January 2021), 594 patients received MAb treatment and 5536 did not. The primary outcome occurred in 75 (12.6%) MAb recipients, 1018 (18.4%) contemporaneous controls, and 1525 (20.6%) historical controls. MAb treatment was associated with decreased likelihood of emergency care or hospitalization (odds ratio, 0.69; 95% CI, 0.60-0.79). After implementation, the weighted probability that a given patient would require an emergency department visit or hospitalization decreased significantly (0.7% per day; 95% CI, 0.03%-0.10%). Mortality was 0.2% (n = 1) in the MAb group compared with 1.0% (n = 71) and 1.0% (n = 57) in pre- and postimplementation controls, respectively. Adverse events occurred in 7 (1.2%); 2 (0.3%) were considered serious.
Conclusions: MAb treatment of high-risk ambulatory patients with early COVID-19 was well tolerated and likely effective at preventing the need for subsequent emergency department or hospital care.
Keywords: COVID-19; SARS-CoV-2; bamlanivimab; casirivimab; imdevimab; monoclonal antibody; novel coronavirus.
© The Author(s) 2021. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

