Traceless Redox-Annulations of Alicyclic Amines
- PMID: 34327302
- PMCID: PMC8318209
- DOI: 10.1055/s-0040-1706004
Traceless Redox-Annulations of Alicyclic Amines
Abstract
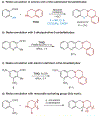
Amines such as 1,2,3,4-tetrahydroisoquinoline undergo redox-neutral annulations with ortho-(nitromethyl)benzaldehyde. Benzoic acid acts as a promoter in these reactions, which involve concurrent amine α-C-H bond and N-H bond functionalization. Subsequent removal of the nitro group provides access to tetrahydroprotoberberines not accessible via typical redox-annulations. Also reported are decarboxylative annulations of ortho-(nitromethyl)benzaldehyde with proline and pipecolic acid.
Keywords: C–H bond functionalization; decarboxylative annulation; denitration; redox-annulation; redox-neutral.
Figures
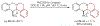


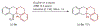

References
-
- Selected recent reviews on amine C–H functionalization, including redox-neutral approaches: Campos KR Chem. Soc. Rev 2007, 36, 1069. - PubMed
- Jazzar R; Hitce J; Renaudat A; Sofack-Kreutzer J; Baudoin O Chem. Eur. J 2010, 16, 2654. - PubMed
- Yeung CS; Dong VM Chem. Rev 2011, 111, 1215. - PubMed
- Mitchell EA; Peschiulli A; Lefevre N; Meerpoel L; Maes BU W. Chem. Eur. J 2012, 18, 10092. - PubMed
- Jones KM; Klussmann M Synlett 2012, 23, 159.
- Peng B; Maulide N Chem. Eur. J 2013, 19, 13274. - PubMed
- Girard SA; Knauber T; Li C-J Angew. Chem. Int. Ed 2014, 53, 74. - PubMed
- Haibach MC; Seidel D Angew. Chem. Int. Ed 2014, 53, 5010. - PubMed
- Wang L; Xiao J Adv. Synth. Catal 2014, 356, 1137.
- Vo C-VT; Bode JW J. Org. Chem 2014, 79, 2809. - PubMed
- Seidel D Org. Chem. Front 2014, 1, 426. - PMC - PubMed
- Qin Y; Lv J; Luo S Tetrahedron Lett 2014, 55, 551.
- Seidel D Acc. Chem. Res 2015, 48, 317. - PMC - PubMed
- Beatty JW; Stephenson CR J. Acc. Chem. Res 2015, 48, 1474. - PMC - PubMed
- Mahato S; Jana CK Chem. Rec 2016, 16, 1477. - PubMed
- Qin Y; Zhu L; Luo S Chem. Rev 2017, 117, 9433. - PubMed
- Cheng M-X; Yang S-D Synlett 2017, 28, 159.
- Chu JCK; Rovis T Angew. Chem. Int. Ed 2018, 57, 62. - PMC - PubMed
- Gonnard L; Guérinot A; Cossy J Tetrahedron 2019, 75, 145.
- Liu S; Zhao Z; Wang Y Chem. Eur. J 2019, 25, 2423. - PubMed
- Antermite D; Bull JA Synthesis 2019, 51, 3171.
- Trowbridge A; Walton SM; Gaunt MJ Chem. Rev 2020, 120, 2613. - PubMed
-
- Recent examples of mechanistically diverse amine C–H bond functionalization reactions: Zhao Z; Luo Y; Liu S; Zhang L; Feng L; Wang Y Angew. Chem. Int. Ed 2018, 57, 3792. - PubMed
- Wang F; Rafiee M; Stahl SS Angew. Chem. Int. Ed 2018, 57, 6686. - PMC - PubMed
- Greßies S; Klauck FJR; Kim JH; Daniliuc CG; Glorius F Angew. Chem. Int. Ed 2018, 57, 9950. - PubMed
- Griffiths RJ; Kong WC; Richards SA; Burley GA; Willis MC; Talbot EP A. Chem. Sci 2018, 9, 2295. - PMC - PubMed
- Idiris FIM; Majeste CE; Craven GB; Jones CR Chem. Sci 2018, 9, 2873. - PMC - PubMed
- Li S-S; Lv X; Ren D; Shao C-L; Liu Q; Xiao J Chem. Sci 2018, 9, 8253. - PMC - PubMed
- Maier AFG; Tussing S; Zhu H; Wicker G; Tzvetkova P; Flörke U; Daniliuc CG; Grimme S; Paradies J Chem. Eur. J 2018, 24, 16287. - PubMed
- Mori K; Isogai R; Kamei Y; Yamanaka M; Akiyama TJ Am. Chem. Soc 2018, 140, 6203. - PubMed
- Shang M; Chan JZ; Cao M; Chang Y; Wang Q; Cook B; Torker S; Wasa MJ Am. Chem. Soc 2018, 140, 10593. - PMC - PubMed
- Lennox AJJ; Goes SL; Webster MP; Koolman HF; Djuric SW; Stahl SS J. Am. Chem. Soc 2018, 140, 11227. - PMC - PubMed
- Zhang J; Park S; Chang SJ Am. Chem. Soc 2018, 140, 13209. - PubMed
- Nauth AM; Schechtel E; Dören R; Tremel W; Opatz TJ Am. Chem. Soc 2018, 140, 14169. - PubMed
- Jiang H-J; Zhong X-M; Yu J; Zhang Y; Zhang X; Wu Y-D; Gong L-Z Angew. Chem. Int. Ed 2019, 58, 1803. - PubMed
- Ashley MA; Yamauchi C; Chu JCK; Otsuka S; Yorimitsu H; Rovis T Angew. Chem. Int. Ed 2019, 58, 4002. - PMC - PubMed
- Guin S; Dolui P; Zhang X; Paul S; Singh VK; Pradhan S; Chandrashekar HB; Anjana SS; Paton RS; Maiti D Angew. Chem. Int. Ed 2019, 58, 5633. - PubMed
- Whitehurst WG; Blackwell JH; Hermann GN; Gaunt MJ Angew. Chem. Int. Ed 2019, 58, 9054. - PubMed
- Ma Y; Yao X; Zhang L; Ni P; Cheng R; Ye J Angew. Chem. Int. Ed 2019, 58, 16548. - PubMed
- Grainger R; Heightman TD; Ley SV; Lima F; Johnson CN Chem. Sci 2019, 10, 2264. - PMC - PubMed
- Vasu D; Fuentes de Arriba AL; Leitch JA; de Gombert A; Dixon DJ Chem. Sci 2019, 10, 3401. - PMC - PubMed
- Asako S; Ishihara S; Hirata K; Takai KJ Am. Chem. Soc 2019, 141, 9832. - PubMed
- Lin W; Zhang K-F; Baudoin O Nat. Catal 2019, 2, 882. - PMC - PubMed
- Chan JZ; Chang Y; Wasa M Org. Lett 2019, 21, 984. - PMC - PubMed
- Zhou L; Shen Y-B; An X-D; Li X-J; Li S-S; Liu Q; Xiao J Org. Lett 2019, 21, 8543. - PubMed
- Kataoka M; Otawa Y; Ido N; Mori K Org. Lett 2019, 21, 9334. - PubMed
- Lee M; Adams A; Cox PB; Sanford MS Synlett 2019, 30, 417. - PMC - PubMed
- Kapoor M; Chand-Thakuri P; Maxwell JM; Liu D; Zhou H; Young MC Synlett 2019, 30, 519.
- Ohmatsu K; Suzuki R; Furukawa Y; Sato M; Ooi T ACS Catal 2020, 10, 2627.
- Roque JB; Kuroda Y; Jurczyk J; Xu L-P; Ham JS; Göttemann LT; Roberts CA; Adpressa D; Saurí J; Joyce LA; Musaev DG; Yeung CS; Sarpong R ACS Catal 2020, 10, 2929. - PMC - PubMed
- Rand AW; Yin H; Xu L; Giacoboni J; Martin-Montero R; Romano C; Montgomery J; Martin R ACS Catal 2020, 10, 4671.
- Liu W; Babl T; Röther A; Reiser O; Davies HM L. Chem. Eur. J 2020, 26, 4236. - PMC - PubMed
- Verma P; Richter JM; Chekshin N; Qiao JX; Yu J-QMM; Koronkiewicz B; Chen S; Houk KN; Mayer JM; Ellman JA J. Am. Chem. Soc 2020, 142, 8194. - PMC - PubMed
- Feng K; Quevedo RE; Kohrt JT; Oderinde MS; Reilly U; White MC Nature 2020, 580, 621. - PMC - PubMed
- Sarver PJ; Bacauanu V; Schultz DM; DiRocco DA; Lam Y.-h.; Sherer EC; MacMillan DWC. Nat. Chem 2020, 12, 459. - PubMed
- McManus JB; Onuska NPR; Jeffreys MS; Goodwin NC; Nicewicz DA Org. Lett 2020, 22, 679. - PubMed
- Oeschger R; Su B; Yu I; Ehinger C; Romero E; He S; Hartwig J Science 2020, 368, 736. - PMC - PubMed
- Short MA; Blackburn JM; Roizen JL Synlett 2020, 31, 102. - PMC - PubMed
-
- Zhang C; De C K; Mal R; Seidel DJ Am. Chem. Soc 2008, 130, 416. - PubMed
- Zheng L; Yang F; Dang Q; Bai X Org. Lett 2008, 10, 889. - PubMed
- Dieckmann A; Richers MT; Platonova AY; Zhang C; Seidel D; Houk KN J. Org. Chem 2013, 78, 4132. - PMC - PubMed
- Richers MT; Deb I; Platonova AY; Zhang C; Seidel D Synthesis 2013, 45, 1730. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
