Bis(N-picolinamido)cobalt(II) Complexes Display Antifungal Activity toward Candida albicans and Aspergillus fumigatus
- PMID: 34327861
- PMCID: PMC8597028
- DOI: 10.1002/cmdc.202100159
Bis(N-picolinamido)cobalt(II) Complexes Display Antifungal Activity toward Candida albicans and Aspergillus fumigatus
Abstract
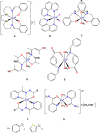
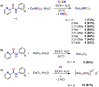
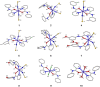
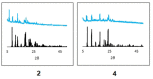
This report highlights the synthesis and characterization of ten new bis(N-picolinamido)cobalt(II) complexes of the type [(L)2 CoX2 ]0/2+ , whereby L=N-picolinamide ligand and X=diisothiocyanato (-NCS), dichlorido (-Cl) or diaqua (-OH2 ) ligands. Single crystal X-ray (SC-XRD) analysis for nine of the structures are reported and confirm the picolinamide ligand is bound to the Co(II) center through a neutral N,O binding mode. With the addition of powder X-ray diffraction (PXRD), we have confirmed the cis and trans ligand arrangements of each complex. All complexes were screened against several fungal species and show increased antifungal activity. Notably, these complexes had significant activity against strains of Candida albicans and Aspergillus fumigatus, with several compounds exhibiting growth inhibition of >80 %, and onecompound inhibiting Aspergillus fumigatus hyphal growth by >90 %. Conversely, no antifungal activity was exhibited toward Cryptococcus neoformans and no cytotoxicity towards mammalian cell lines.
Keywords: Aspergillus fumigatus; Bioinorganic Chemistry; Candida albicans; Cobalt Complexes; Picolinamide ligands.
© 2021 The Authors. ChemMedChem published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Bongomin F., Gago S., Oladele R. O., Denning D. W., J. Fungi 2017, 3, 57.
-
- Andriole V. T., J. Antimicrob. Chemother. 1999, 44, 151–162. - PubMed
-
- Brown G. D., Denning D. W., Gow N. A. R., Levitz S. M., Netea M. G., White T. C., Sci. Transl. Med. 2012, 4, 165rv13. - PubMed
-
- Forsberg K., Woodworth K., Walters M., Berkow E. L., Jackson B., Chiller T., Vallabhaneni S., Med. Mycol. 2019, 57, 1–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

