Leveraging Health Information Technology to Collect Family Cancer History: A Systematic Review and Meta-Analysis
- PMID: 34328789
- PMCID: PMC8812651
- DOI: 10.1200/CCI.21.00004
Leveraging Health Information Technology to Collect Family Cancer History: A Systematic Review and Meta-Analysis
Abstract
Purpose: Collection of family cancer histories (FCHs) can identify individuals at risk for familial cancer syndromes. The aim of this study is to evaluate the literature on existing strategies whereby providers use information technology to assemble FCH.
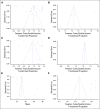
Methods: A systematic search of online databases (Ovid MEDLINE, Cochrane, and Embase) between 1980 and 2020 was performed. Statistical heterogeneity was assessed through the chi-square test (ie, Cochrane Q test) and the inconsistency statistic (I2). A random-effects analysis was used to calculate the pooled proportions and means.
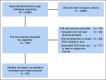
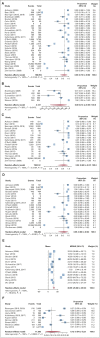
Results: The comprehensive search produced 4,005 publications. Twenty-eight studies met inclusion criteria. Twenty-seven information technology tools were evaluated. Eighteen out of 28 studies were electronic surveys administered before visits (18, 64.3%). Five studies administered tablet surveys in offices (5, 17.8%). Four studies collected electronic survey via kiosk before visits (4, 14.3%), and one study used animated virtual counselor during visits (1, 3.6%). Among the studies that use an FCH tool, the pooled estimate of the overall completion rate was 86% (CI, 72% to 96%), 84% (CI, 65% to 97%) for electronic surveys before visits, 89% (CI, 0.74 to 0.98) for tablet surveys, and 85% (CI, 0.66 to 0.98) for surveys via kiosk. Mean time required for completion was 31.0 minutes (CI, 26.1 to 35.9), and the pooled estimate of proportions of participants referred to genetic testing was 12% (CI, 4% to 23%).
Conclusion: Our review found that electronic FCH collection can be completed successfully by patients in a time-efficient manner with high rates of satisfaction.
Conflict of interest statement
Figures

References
-
- Daly MB, Pilarski R, Yurgelun MB, et al. NCCN guidelines insights: Genetic/familial high-risk assessment: Breast, ovarian, and pancreatic, version 1.2020 J Natl Compr Canc Netw 18380–3912020 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

