Histone H2A.X phosphorylation and Caspase-Initiated Chromatin Condensation in late-stage erythropoiesis
- PMID: 34330317
- PMCID: PMC8325214
- DOI: 10.1186/s13072-021-00408-5
Histone H2A.X phosphorylation and Caspase-Initiated Chromatin Condensation in late-stage erythropoiesis
Abstract
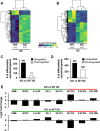
Background: Condensation of chromatin prior to enucleation is an essential component of terminal erythroid maturation, and defects in this process are associated with inefficient erythropoiesis and anemia. However, the mechanisms involved in this phenomenon are not well understood. Here, we describe a potential role for the histone variant H2A.X in erythropoiesis.
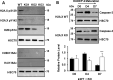
Results: We find in multiple model systems that this histone is essential for normal maturation, and that the loss of H2A.X in erythroid cells results in dysregulation in expression of erythroid-specific genes as well as a nuclear condensation defect. In addition, we demonstrate that erythroid maturation is characterized by phosphorylation at both S139 and Y142 on the C-terminal tail of H2A.X during late-stage erythropoiesis. Knockout of the kinase BAZ1B/WSTF results in loss of Y142 phosphorylation and a defect in nuclear condensation, but does not replicate extensive transcriptional changes to erythroid-specific genes observed in the absence of H2A.X.
Conclusions: We relate these findings to Caspase-Initiated Chromatin Condensation (CICC) in terminal erythroid maturation, where aspects of the apoptotic pathway are invoked while apoptosis is specifically suppressed.
Keywords: Apoptosis; BAZ1B; Caspase; Chromatin condensation; Erythropoiesis; H2A.X; Phosphorylation; Terminal erythroid maturation; WSTF; g-H2A.X.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

