Mechanisms of regulatory T cell infiltration in tumors: implications for innovative immune precision therapies
- PMID: 34330764
- PMCID: PMC8327843
- DOI: 10.1136/jitc-2021-002591
Mechanisms of regulatory T cell infiltration in tumors: implications for innovative immune precision therapies
Erratum in
-
Correction: Mechanisms of regulatory T cell infiltration in tumors: implications for innovative immune precision therapies.J Immunother Cancer. 2021 Sep;9(9):e002591corr1. doi: 10.1136/jitc-2021-002591corr1. J Immunother Cancer. 2021. PMID: 34518293 Free PMC article. No abstract available.
Abstract
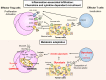
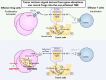
With the broad application of cancer immunotherapies such as immune checkpoint inhibitors in multiple cancer types, the immunological landscape in the tumor microenvironment (TME) has become enormously important for determining the optimal cancer treatment. Tumors can be immunologically divided into two categories: inflamed and non-inflamed based on the extent of immune cell infiltration and their activation status. In general, immunotherapies are preferable for the inflamed tumors than for non-inflamed tumors. Regulatory T cells (Tregs), an immunosuppressive subset of CD4+ T cells, play an essential role in maintaining self-tolerance and immunological homeostasis. In tumor immunity, Tregs compromise immune surveillance against cancer in healthy individuals and impair the antitumor immune response in tumor-bearing hosts. Tregs, therefore, accelerate immune evasion by tumor cells, leading to tumor development and progression in various types of cancer. Therefore, Tregs are considered to be a crucial therapeutic target for cancer immunotherapy. Abundant Tregs are observed in the TME in many types of cancer, both in inflamed and non-inflamed tumors. Diverse mechanisms of Treg accumulation, activation, and survival in the TME have been uncovered for different tumor types, indicating the importance of understanding the mechanism of Treg infiltration in each patient when selecting the optimal Treg-targeted therapy. Here, we review recent advances in the understanding of mechanisms leading to Treg abundance in the TME to optimize Treg-targeted therapy. Furthermore, in addition to the conventional strategies targeting cell surface molecules predominantly expressed by Tregs, reagents targeting molecules and signaling pathways specifically employed by Tregs for infiltration, activation, and survival in each tumor type are illustrated as novel Treg-targeted therapies. The effectiveness of immune precision therapy depends on conditions in the TME of each cancer patient.
Keywords: CD4-positive T-lymphocytes; costimulatory and inhibitory molecules; immunotherapy; tumor microenvironment.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: SK received research funding from Ono Pharmaceutical and Bristol-Myers Squibb outside this study. HN received honoraria and research funding from Ono Pharmaceutical, Chugai Pharmaceutical, MSD, and Bristol-Myers Squibb, and research funding from Taiho Pharmaceutical, Daiichi-Sankyo, Kyowa Kirin, Zenyaku Kogyo, Oncolys BioPharma, Debiopharma, Asahi-Kasei, Sysmex, Fujifilm, SRL, Astellas Pharmaceutical, Sumitomo Dainippon Pharma, and BD Japan outside this study.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
