Secukinumab provides sustained improvement in signs and symptoms and low radiographic progression in patients with psoriatic arthritis: 2-year (end-of-study) results from the FUTURE 5 study
- PMID: 34330846
- PMCID: PMC8327842
- DOI: 10.1136/rmdopen-2021-001600
Secukinumab provides sustained improvement in signs and symptoms and low radiographic progression in patients with psoriatic arthritis: 2-year (end-of-study) results from the FUTURE 5 study
Abstract
Objective: Secukinumab provided sustained efficacy, low radiographic progression and consistent safety over 52 weeks in patients with psoriatic arthritis (PsA) in the FUTURE 5 study. Here, we report 2-year (end-of-study) results from this study.
Methods: Adults with active PsA were randomised 2:2:2:3 to receive subcutaneous secukinumab 300 mg load (300 mg), 150 mg load (150 mg), 150 mg no load or placebo at baseline; weeks 1, 2, 3 and 4; and every 4 weeks thereafter. Secukinumab could be escalated from 150 mg to 300 mg starting at week 52, if active signs of disease were observed based on physician's assessment. Assessments at week 104 (2 years) included clinical end points and radiographic damage (mean change in van der Heijde-modified total Sharp score (vdH-mTSS)). Safety analysis included all patients who received ≥1 dose of study medication.
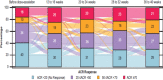
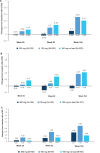
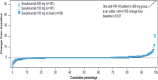
Results: Of the 996 patients randomised, 783 patients (78.6%) completed 2 years of treatment. Improvement in clinical end points was sustained through 2 years. The vdH-mTSS (mean change (SD)) was 0.10 (1.74; 300 mg), 0.52 (2.66; 150 mg) and 0.41 (2.20; 150 mg no load) at 2 years. The proportion of patients with no radiographic progression (change from baseline in vdH-mTSS ≤0.5) at 2 years was 89.5% (300 mg), 82.3% (150 mg) and 81.1% (150 mg no load).
Conclusion: Secukinumab with and without loading regimen provided sustained clinical efficacy and low radiographic progression through 2 years in patients with PsA. No new safety findings were reported.
Trial registration number: NCT02404350.
Keywords: arthritis; biological therapy; cytokines; inflammation; psoriatic.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: PJM has received research grants, consulting and speaking fees from AbbVie, Amgen, Eli Lilly, Janssen, Novartis, Pfizer and UCB; research grants and consulting fees from Bristol-Myers Squibb, Galapagos, Gilead and Sun; and consulting fees from Boehringer Ingelheim and GlaxoSmithKliine. RBL has received consulting fees from AbbVie, Novartis, EliLilly, UCB, Pfizer, Galapagos and Gilead. PR has received research grants from Janssen and Novartis, and consulting and speaking fees from AbbVie, Amgen, BMS, Celgene, Eli Lilly, Janssen, Merck, Novartis, Pfizer and UCB. HT, AS and EB have nothing to disclose. SN has received research grants from Novartis and Johnson & Johnson, and consulting fees from Novartis, Johnson & Johnson and Pfizer. AR is an employee of Novartis, with Novartis stock. SM is an employee of Novartis, with Novartis stock. EMD was an employee of Novartis and LP is an employee of Novartis. DvdH has received consulting fees from AbbVie, Amgen, Astellas, AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Celgene, Cyxone, Daiichi, Eisai, Eli-Lilly, Galapagos, Gilead, Glaxo-Smith-Kline, Janssen, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi, Takeda and UCB Pharma. DvdH is a Director of Imaging Rheumatology
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
